Copyright
©The Author(s) 2020.
World J Biol Chem. Sep 27, 2020; 11(2): 52-61
Published online Sep 27, 2020. doi: 10.4331/wjbc.v11.i2.52
Published online Sep 27, 2020. doi: 10.4331/wjbc.v11.i2.52
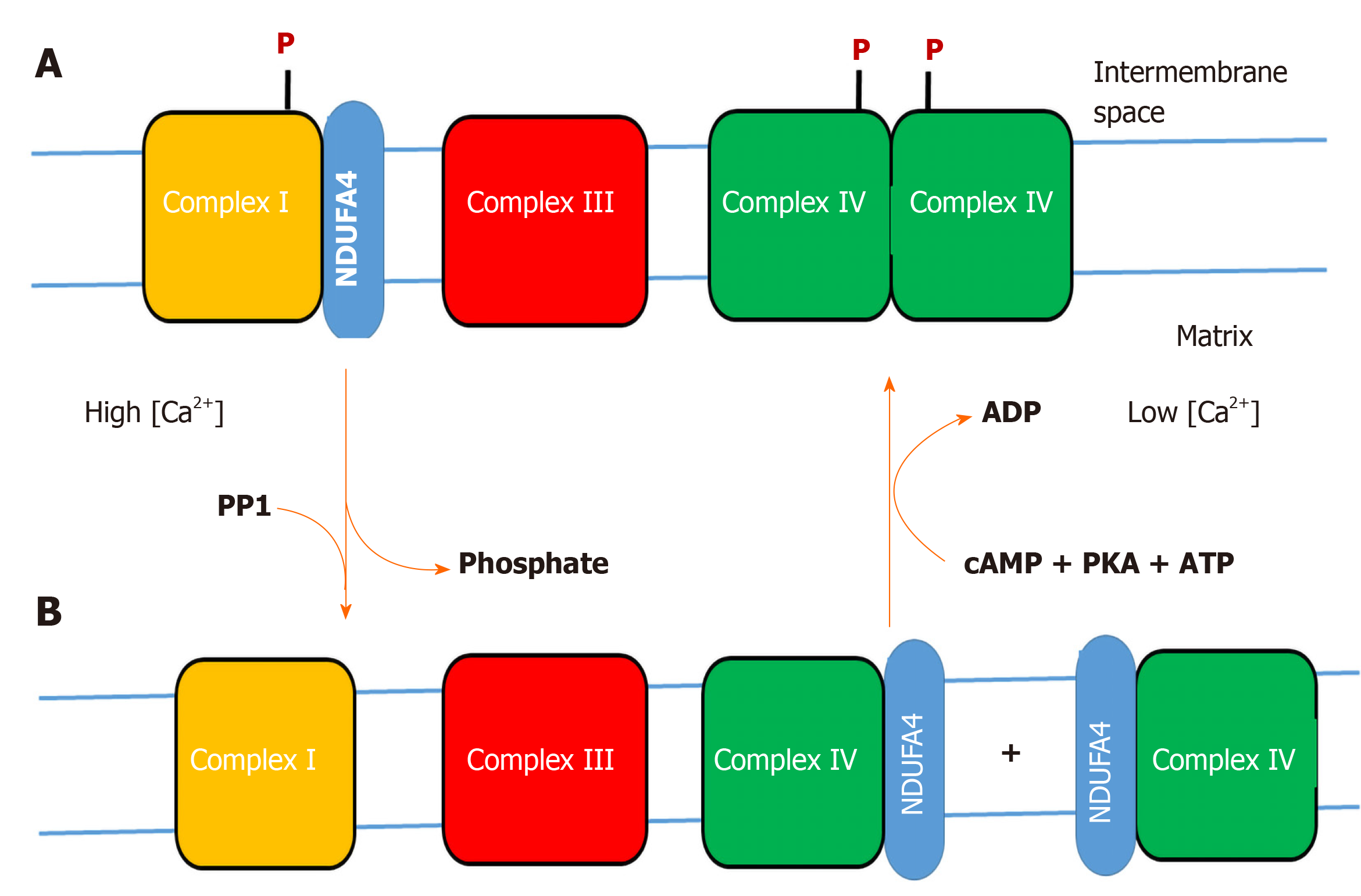
Figure 1 Hypothesis on the variable binding of NDUFA4 to complex I or cytochrome c oxidase.
A: Phosphorylation (P) of complex I and cytochrome c oxidase (CytOx) at low cytosolic calcium (< 1 micromolar) stabilizes binding of NDUFA4 to complex I and the function of the “allosteric ATP-inhibition” of dimeric CytOx (resting state); B: Stress-induced increase of cytosolic calcium (> 1 micromolar) dephosphorylates complex I and CytOx by a calcium-activated PP1, accompanied by monomerization of CytOx and switching off its allosteric ATP-inhibition. NDUFA4 is detached from complex I and binds to monomeric CytOx (excited state). At low cytosolic calcium, a cAMP-dependent protein kinase A phosphorylates complex I and CytOx. This changes the binding position of NDUFA4 from CytOx to complex I, accompanied by dimerization of CytOx and activation of its allosteric ATP-inhibition.
- Citation: Kadenbach B. Regulation of cytochrome c oxidase contributes to health and optimal life. World J Biol Chem 2020; 11(2): 52-61
- URL: https://www.wjgnet.com/1949-8454/full/v11/i2/52.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v11.i2.52