BACKGROUND
The objective of robotic surgery is allowing us to operate in challenging environments or to achieve levels of performance we would otherwise not be capable of. Surgeons interact with their environment by using their senses to gather information (perception), combining these inputs with their pre-existing knowledge and experience (processing) to change the environment (action). A robot may augment any or all of these aspects in order to improve the final outcome.
Over the last two decades colorectal surgery has dramatically changed due to the widespread implementation of laparoscopic surgery. Laparoscopic surgery offers comparable oncological outcomes[1], but with improved post-operative recovery[2]. The move towards minimal access surgery has, however, put challenges upon the surgeon’s perceptive and action abilities with a resultant increased reliance on processing abilities required to make up for these deficits. Robotic assistance in minimal access surgery aims to make up for some of the practical shortcomings of laparoscopic surgery, providing assistance to the surgeon with improvements to perception, processing and action.
This aim of this review is to summarise the current benefits and shortcomings of robotics in colorectal surgery and endoscopy and to identify how the implementation of developing robotic technology may shape the future of colorectal surgery.
ESTABLISHED ROBOTIC COLORECTAL SURGERY
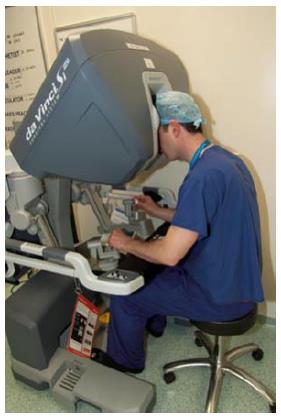
At present the Da Vinci Robot (DVR) (Intuitive Surgical) is the most widely used platform for robot assisted laparoscopic colorectal surgery. It consists of a high definition three-dimensional camera system allied to a patient “sidecart” that allows instruments to be delivered and controlled. The surgeon sits at a separate control module (Figure 1) that delivers three-dimensional images and allows remote control of the sidecart-mounted effectors.
Figure 1 Surgeon interaction with the Da Vinci robot control module.
The DVR addresses some of the limitations of conventional laparoscopic surgery by allowing dexterity in 7 planes of movement within a limited space, static ports, filtering of physiological tremor and variable motion scaling. The potential drawbacks of the system include lack of tactile feedback, prolonged operative time and financial cost, including initial outlay, consumables and servicing of equipment.
The attributes of the DVR make it suitable for assisting in precision surgery within confined spaces such as the pelvis and use of the DVR for radical prostatectomy is now widespread in the United Kingdom for this reason. Robotic prostatectomy is now seen as the primary treatment for localised prostate cancer, delivering equivalent oncological outcomes with decreased morbidity[3,4], but equivocal improvement in sexual function[5].
The practical challenges of pelvic surgery for prostate cancer are similar to those encountered in rectal surgery, particularly when performing total mesorectal excision (TME). It has been demonstrated that laparoscopic TME offers equivalent oncological outcome with faster recovery and less morbidity than open surgery[2,6]. However, it is technically demanding with higher conversion rates seen in the obese and during low rectal surgery[7].
Comparative studies have suggested an improved TME grade following robotic TME[8,9] and it is hypothesised that the improved precision of surgery enables the TME plane to be more accurately preserved, offering greater preservation of the pelvic autonomic nerves resulting in improved urinary and sexual function with some evidence of short-term benefit[10]. Rates of conversion are often used as a surrogate marker of operative difficulty and a systematic review of case-controlled studies identified that conversion rates may be lower in robotic assisted cases, although this was not statistically significant[11].
The Robotic vs Laparoscopic Resection for Rectal cancer trial is the first international, multi-centre randomised controlled trial to compare laparoscopic with robotic TME. The results of 471 participants have been presented at the European Society of Coloproctology, September 2015 and demonstrated no statistically significant difference in oncological clearance, patient outcome or conversion to open surgery between the two groups. These findings may impact the usage of the DVR in TME as it seems that the increased financial cost of robotic usage is not offset by improved surgical outcomes.
There are several centers performing robotic ventral mesh rectopexy. It has been argued that the increased dexterity of the instruments of the DVR facilitates dissection and more precise suturing of the mesh[12]. There are few studies that compare outcomes between laparoscopic and robotic ventral mesh rectopexy, however functional improvements with respect to obstructive defaecation symptoms have been noted in patients having robotic surgery[13].
The COST and COLOR trials demonstrated that laparoscopic surgery offers oncological and survival outcomes commensurate with open surgery in colonic tumors[14,15]. Decreased morbidity and length of hospital stay have also been shown[1,16,17]. The improved dexterity the robot offers has demonstrable benefit when performing intracorporeal anastomosis[18], but the benefits of robotic over laparoscopic colonic surgery however are less well established and no benefit of has been demonstrated when comparing laparoscopic to robotic right hemicolectomy[19]. Robotic left hemicolectomy can be used as training platform to practice mobilization of the left colon and splenic flexure as part of robotic anterior resection.
Currently there appears to be little evidence to support the use of DVR type robots in conventional multiport trans-abdominal surgery. The development of transanal endoscopic microsurgery (TEM) and single port laparoscopic surgery (SPLS) limits a surgeons dexterity still further and these fields may be particularly suited to robotic augmentation.
COLORECTAL ROBOTIC SURGERY UNDER DEVELOPMENT
Robotic surgical technology is expensive- initial outlay, maintenance and purchasing disposable equipment contributes to the financial expense that must be justified by reproducible cost effectiveness. This currently restricts robotic surgery to larger institutions that are able to absorb these costs and provide high utilisation in circumstances where financial gains can offset expenditure. Therefore developments in colorectal robotics over the next decade will concentrate on the widening application of robotics to other colorectal disciplines, such as endoscopy, single port surgery and transanal surgery and minimisation of cost.
Single port robotic laparoscopic surgery
SPLS offers improved cosmesis and less post-operative discomfort compared to that seen in multiport laparoscopic surgery[20]. SPLS restricts the triangulation and retraction easily achieved in multiport surgery. This can be managed utilising conventional straight instruments that are crossed intracorporeally or by using curved or articulating instruments such as the Autonomy Lapro-angle[20]. The technique is associated with equivalent oncological outcomes[21], but a systematic review of colorectal SPLS found conflicting evidence regarding demonstrable improvements in patient recovery and length of stay[22].
Robotic assisted SPLS systems offer superior triangulation, without the need for crossing instruments, while incorporating other robotic technology. The Da Vinci Si Surgical Robot (Intuitive Surgical) has obtained FDA approval. It incorporates remote centre technology that reduces interference between instruments in addition to a three dimensional camera, motion scaling and removal of tremor (Figure 2). Evidence has been published demonstrating the feasibility of robot assisted SPLS in right hemicolectomy[23] but to date no advantage of robot assisted over conventional SPLS has been demonstrated. Alternative robotic SPLS platforms are in the prototype stage, including the Single Port Orifice Robotic Technology robotic SPLS module (Titan Medical) which is predicted to cost a third of the Da Vinci system, although data on efficacy is awaited[24].
Figure 2 Robotic single port laparoscopic surgery module designed by Intuitive Surgical.
©[2015] Intuitive Surgical, Inc.
Robotic transanal surgery
TEM offers similar practical challenges to SPLS and has become established as an effective method of removing non-advanced distal rectal lesions and may be oncologically superior to conventional transanal excision[25]. Initially described in 2011, a SILS (Covidien) port was placed in the anus and the Da Vinci machine deployed as for SPLS[26]. The first cohort study of sixteen patients managed with robotic TEM reported that the procedure was technically feasible but did not offer comparative data with conventional TEM. Use of the robot added an additional €1000 per procedure in disposables alone[27].
Robotic transanal total mesorectal excision (RT-TME) is an alternative method of TME where the standard abdominal component of an anterior resection is completed laparoscopically before the DVR is introduced transanally to complete the TME in a retrograde fashion. This method facilitates distal rectal dissection in patients who are obese or who have narrow pelvises[28]. A study of twenty patients did not compare RT-TME to conventional TME but demonstrated the feasibility of the approach in distal rectal cancers[29].
Improving current laparoscopic technology
Modifying and augmenting existing laparoscopic surgical instrumentation to offer additional degrees of movement, tactile or haptic feedback may narrow the gulf between laparoscopic and robotic surgery with potentially significant cost savings. Movement of conventional laparoscopic instruments is restricted by the fulcrum of movement existing at the point of entry into the abdomen. The Radius Surgical System (RSS, Tuebingen Scientific) has been in circulation for over ten years and offers an additional fulcrum at the tip of the instrument to allow a greater degree of movement. As with the DVR, it offers 7 degrees of freedom for a significantly lower financial outlay although the extent of its distal joint articulation is reduced[30].
The RSS generally offers tools for suturing and manipulation, rather than dissection and there is no mechanism for removing surgeon tremor or changing the ratio of hand to instrument movement. Results suggest a shorter learning curve compared to the DVR[31] and it has been demonstrated that they can be used in sutured intracorporeal colorectal anastomosis with encouraging results[32]. The parallel development of reliable laparoscopic stapling devices has, however, generally obviated the need for an advanced suturing instrument, which may account for the lack of take up of the RSS system. The Autonomy Laparo-angle (CambridgeEndo) is a simpler system offering a range of graspers, scissors and needle holders that can articulate at a distal joint allowing a greater degree of movement not offered by conventional laparoscopic instruments. However, to date there is no published evidence of its use in colorectal surgery.
Robotic endoscopy
Developments in robotic endoscopy have focused on automatic endoscope propulsion and improved endoscopic instrumentation. Balloon endoscopy mimics the movement of an earthworm, using coordinated inflation and deflation of a series of balloon to advance the camera and it has been successful in small bowel enteroscopy[33]. Current research focuses on providing propulsion at the endoscopic tip to pull the scope through the colon, reducing discomfort and procedure duration. Development of legged locomotion allows efficient propulsion and a steering capability, but risks iatrogenic injury from the traction of the legs on the colonic surface. New generations of microscopic leg effectors aim to minimise injury while offering sufficient propulsive force for effective motion[34].
Capsule endoscopy utilises passive propulsion to traverse the GI tract and has proved successful in endoscopic practice, particularly in visualisation of the small bowel. The purely passive locomotion is also a drawback and does not allow retrograde motion to recheck areas of incomplete examination. Capsules with active control could be steered to closely examine certain areas, release medications and perform diagnostic or therapeutic interventions but they are limited by size as they must be swallowed. Size constraints currently preclude independently self-propelled capsules.
Vectoring using external magnets allows the capsule to remain as small as possible and was originally described using a hand-controlled external magnet[35]. To offer accurate and reproducible magnetic control requires a generated magnetic field utilising a series of magnets under computer control and offering very high positional accuracy at the cost of extensive magnetic equipment[36]. Early trials demonstrate that the technique is feasible but movement is restricted by collapsed bowel with no method of insufflation available and there are no reports from trials in human subjects[37].
Endoscopic mucosal resection is an established method of endoscopic piecemeal removal of sessile polyps or superficial cancers less than 20 mm in diameter that would otherwise require surgical excision[38]. Endoscopic submucosal resection (ESR) can be applied to larger lesions and aims to remove a greater depth of tissue in a single specimen. This allows more accurate histological examination and reduces the risk of recurrent disease[39]. Although initially developed for the management of upper gastrointestinal lesions, the procedure has shown great promise with respect to colonic lesions greater than two centimeters in diameter[40]. The procedure is technically challenging and relies on the application of tension to the target lesion to allow careful dissection, which is challenging with conventional endoscopic instruments.
A number of flexible multi-tasking platforms are available that consist of conventional endoscope video technology with an enhanced multichannel intervention system allowing two working instruments operated mechanically or robotically. The Master and Slave Transluminal Endoscopic Tool (MASTER) is a robotic endoscopic surgical system that introduces a two-channel endoscope with two slave robotic effectors possessing nine degrees of freedom. The system allows separation of the endoscopic control and instrument control to allow two operators to work together in tandem[41]. The MASTER was originally developed for Natural Orifice Transluminal Endoscopic Surgery (NOTES) but has been tested in ESR in animal models with success[42]. A trial of the MASTER system in ESR in human subjects was planned but results have not been published yet.
POTENTIAL FUTURE OF ROBOTIC COLORECTAL SURGERY
The ultimate aim of minimal access surgery is for surgery to be completed via natural orifices without any disruption to the normal functioning of the patient. Ultimately the development of nanotechnology may make this a reality but in the meantime the direction of minimal access surgery is to further minimise access without compromising surgical outcome and to improve patient safety.
Advanced instrumentation
The Image-Sensing Navigated and Kinematically Enhanced (i-SNAKE) is an instrument delivered via a standard laparoscopic port. The distal end of the instrument possesses an articulated section carrying a camera, driven by a hybrid motor design, allowing a greater degree of flexion compared to cable actuators used in a conventional flexible endoscope[43]. In addition, there are two flexible surgical arms driven by cables that can carry a range of instruments and there is an additional channel that allows an instrument to be passed through the articulated section. The three arms are delivered via a 15 mm trocar and the arms are extended once safely within the peritoneal cavity.
Flexible robots such as this are required to operate in highly angulated positions while maintained sufficient control to allow careful dissection and to produce enough force to manipulate tissue. The i-SNAKE can retroflex completely, allowing tubal ligation from a vaginal NOTES approach[44]. The suitability of the platform for conducting intraluminal interventions such as ESR and Per-Oral Endoscopic Myotomy have been assessed[45] and it is would be anticipated that this technology could be used to augment SPLS and intraluminal colorectal interventions.
Haptic feedback
Haptic feedback describes the conveying of information from the robotic effector, now also functioning as a receptor, back to the surgeon. The aim is to provide “transparency”, where the surgeon feels that they are contacting the patient directly, rather than via a robotic mechanism[46]. To achieve this level of feedback requires transmission of information regarding temperature, texture, force and vibration, and may not be technically feasible. Other industries make use of limited forms of haptic feedback, but surgery offers the unique challenges of size limitation, sterilisability and cost implications over existing technology. An economical approach would be to modify existing technology with feedback sensors and effectors[47], but this may make integration with complex technology such as the DVR challenging.
Colorectal surgery demands soft tissue differentiation, the careful manipulation of tissues and suturing, all of which benefit from haptic feedback. The TELELAP ALF-X (SOFAR) is a surgical robot that offers haptic feedback in a smaller package compared to the DVR[48]. The TELELAP ALF-X provides haptic feedback by exerting forces on the surgeon’s hands- this requires a complex system of processors and actuators to achieve adequate fidelity and is therefore inherently complex. An alternative approach would be to relay haptic information to the surgeon by an auditory or visual representation of force feedback. Lab studies have demonstrated that color-coded visual display of stitch tension improves consistency in tension applied to ligatures[49,50].
To provide haptic feedback in the seven degrees of freedom that the DVR offers would be even more challenging. Given the wide uptake of the DVR it may be unlikely that institutions will invest in another robot simply to take advantage of haptic feedback when the surgeon feels it may be helpful. Therefore a successful haptic feedback system would most likely have to be integrated with the DVR, or operate in parallel with it.
A force-sensing adaptor for the Da Vinci Robot has been developed with some success in lab testing but there are no data from in vivo tests[51]. A wireless palpation probe is an alternative that could be used both in robotics and conventional laparoscopic surgery. This battery operated unit can be introduced via a port and used to measure indentation pressure and depth in order to characterise tissues. Initial porcine studies used the probe to serially palpate a porcine liver to produce a “stiffness map” that could be used to guide subsequent resection[52].
Tactility
Open surgery offers a uniquely tactile experience that is significantly dampened by minimally invasive surgery. Haptic feedback may offer some gross information on tissue resistance, but not the degree that the surgeon requires for accurate tissue differentiation. The technology to provide tactile transparency does not currently exist and may not do so for some time due to the technical complexity of detecting, processing and displaying such information.
Instruments for the detection of gross tactile information in minimal access surgery have been developed and tested in order to locate arteries and detect blood flow, in identifying the inferior mesenteric artery for example. The tools, such as TactArray (Pressure Profile Systems) carry multiple pressure sensitive receptors that may be applied to the tissues producing graphic representations of the tactile feedback detected[53]. An alternate approach would be to use intracorporeal Doppler ultrasonography as a proxy to assess the tissue instead or relying on tactile feedback. This has already been demonstrated in laparoscopic nephrectomy[54] but has not been utilised in colorectal surgery.
Capsule endoscopy
Diagnostic capsule endoscopy has proven itself as a diagnostic modality and is already in widespread use. Further development of this technology in the future will look to expand its diagnostic and therapeutic possibilities. Wireless capsule endoscopy is limited by the lack of a conventional insufflation system and the resultant lack of bowel distension limits diagnostic capability. Preliminary studies have demonstrated the feasibility of a wireless insufflation capsule utilising liquids or powders to produce gaseous insufflation[55]. There have been no published results from animal studies but this demonstrates the widening functionality of capsule intervention.
As capsule endoscopes become more complex they will become limited by their power storage capacity. Complex luminal processes such as delivery of topical chemotherapy, brachytherapy or treatments for inflammatory bowel disease may require capsules to remain inside the body for a prolonged period of time. Therefore an alternate source of power may be necessary. Magnetic induction offers a method of transmitting power to a device within the body from an external charger over a prolonged period of time without causing deleterious effects[56].
Micro- and nanotechnology
Nanotechnology offers opportunities to investigate and intervene at the cellular level and may ultimately lead to a step change in the conduct of colorectal surgery over the next fifty to one hundred years. Currently machine actuators limit the minimum size of machines to a few milimetres- too large to be injected into the circulation for example, but alternative avenues for micro-interventions have been investigated.
Minimally invasive biopsy retrieval has been demonstrated using “micro-grippers”. These tools possess minuscule biopsy tools that close in response to a change in temperature and are also composed of a ferromagnetic alloy. These tools were injected into the common bile duct during an endoscopic retrograde cholangio-pancreatography in a live porcine model, allowed to sit for ten minutes to allow the grippers to close in response to change in temperature before being retrieved by a catheter with a magnetic tip[57]. The retrieved tissue was assessed and deemed suitable for cytological analysis. Potentially this technology could be adapted for use as a non-invasive diagnostic aid in colonic conditions such as inflammatory bowel disease.
Nanobot technology in medicine aims to deliver therapy at the cellular level and may have particular importance in the treatment of cancers. Mechanical actuators and processors are not viable at this scale and therefore the robots are genetically engineered bacteria that allow cellular-level interactions, containing ferromagnetic granules that allow steering and propulsion using magnetic fields. Microbot technology and its application in medicine are likely to radically change how we view therapeutics. The ranges of possible applications include targeted therapy, material removal, deployment of structures, such as stents and telemetry[56]. Although currently in its infancy, this aspect of robotic technology has the greatest potential to revolutionise how we manage colorectal pathology in the future.