Published online Feb 27, 2021. doi: 10.4240/wjgs.v13.i2.198
Peer-review started: November 11, 2020
First decision: December 4, 2020
Revised: December 23, 2020
Accepted: January 21, 2021
Article in press: January 21, 2021
Published online: February 27, 2021
The initial operation of choice in many patients presenting as an emergency with ulcerative colitis is a subtotal colectomy with end ileostomy. A percentage of patients do not proceed to completion proctectomy with ileal pouch anal anastomosis.
To review the existing literature in relation to the significant long-term complic-ations associated with the rectal stump, to provide an overview of options for the surgical management of remnant rectum and anal canal and to form a consolidated guideline on endoscopic screening recommendations in this cohort.
A systematic review was carried out in accordance with PRISMA guidelines for papers containing recommendations for endoscopy surveillance in rectal remnants in ulcerative colitis. A secondary narrative review was carried out exploring the medical and surgical management options for the retained rectum.
For rectal stump surveillance guidelines, 20% recommended an interval of 6 mo to a year, 50% recommended yearly surveillance 10% recommended 2 yearly surveillance and the remaining 30% recommended risk stratification of patients and different screening intervals based on this. All studies agreed surveillance should be carried out via endoscopy and biopsy. Increased vigilance is needed in endoscopy in these patients. Literature review revealed a number of options for surgical management of the remnant rectum.
The retained rectal stump needs to be surveyed endoscopically according to risk stratification. Great care must be taken to avoid rectal perforation and pelvic sepsis at time of endoscopy. If completion proctectomy is indicated the authors favour removal of the anal canal using an intersphincteric dissection technique.
Core Tip: Rectal stumps require long term surveillance due to well documented risk of malignancy, the authors provide a summary of current guidance and recommendations for this. Patients may require completion proctectomy due to dysplasia, malignancy or persistent symptoms, options for completion proctectomy are explored. Endoscopic surveillance of the rectal stump poses certain challenges, potential complications and their management are explored.
- Citation: Hennessy O, Egan L, Joyce M. Subtotal colectomy in ulcerative colitis—long term considerations for the rectal stump. World J Gastrointest Surg 2021; 13(2): 198-209
- URL: https://www.wjgnet.com/1948-9366/full/v13/i2/198.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i2.198
Ulcerative colitis is an idiopathic inflammatory condition in which there is relapsing and remitting inflammation involving predominantly the colon and rectum, generally limited to the superficial mucosal layer[1]. There is a rising incidence worldwide, with prevalence highest in Europe and North America[2-4]. While the need for surgical intervention in ulcerative colitis is decreasing with the advent of biologic therapies, a significant proportion of patients still progress to operative management[5-7]. In recent analysis, 10-year rates of colectomy were still found to be as high as 17% in some patient cohorts[8]. It is estimated that 12%-25% of ulcerative colitis patients will require hospitalisation due to severe exacerbation, and the risk of colectomy during these admissions is as high as 20%-30%[9]. A multidisciplinary approach involving gastroenterology, colorectal surgery, stoma therapy, dietetics and other ancillary services is important early in this process.
Total proctocolectomy remains a very safe single stage procedure and is the gold standard for elective management of ulcerative colitis. In patients abhorrent to a permanent ileostomy, creation of an ileal pouch anal anastomosis (IPAA) most often through a staged approach has good long-term outcomes. In episodes of acute severe ulcerative colitis requiring urgent or emergent surgical input a large proportion of patients are initially managed via a subtotal colectomy (STC) and end ileostomy with associated retention of a rectal stump[10-12]. In the acute setting STC offers a safe procedure for patients in the setting of an active inflammatory state, while also preserving the option of future return of intestinal continuity via an IPAA or, less commonly, an ileorectal anastomosis (IRA)[2,11,13]. In addition to an emergency presentation the rectum may also be left in situ due to patient factors including concerns regarding the associated risk of pelvic nerve damage, infertility and sexual function with proctectomy, this is an important consideration in females of child bearing age[14,15].
Of interest, studies from Sweden and the United Kingdom have shown that in some cohorts, only one third to half of patients undergo reconstruction post colectomy[16,17]. This may be due to a number of factors ranging from satisfaction with quality of life post STC, to being unfit for further operative intervention, as well as lack of patient awareness regarding reconstructive options. Unfortunately the retained rectal stump is not without its complications. Patients can suffer from persistent symptoms including ongoing mucous or bloody discharge, low grade fevers and feelings of rectal discomfort or urgency due to ongoing ulcerative proctitis or diversion proctitis[18,19]. Rates of diversion proctitis may be as high as 90% in varying degrees of severity[20]. Symptoms may start as soon as a few months after faecal diversion and in many patients this ongoing discharge has a deleterious effect on their quality of life. In this cohort medical or surgical intervention may be required[21].
Long term, there is also a well-documented risk of malignancy in the retained rectum[22]. A recent meta-analysis by Derikx et al[22] has shown this risk to be as high as 3% in those with a retained rectal stump, vs 1% in those who undergo resection and reconstruction with an ileoanal pouch. For primary IPAA surgery the majority of colorectal surgeons have now moved to a stapled technique when anastomosing the ileal pouch to the anal canal. This is technically easier and associated with improved functional outcome[23]. However, this involves leaving 1-2 cm of rectum in-situ which can give rise to “cuffitis” and long-term potential for malignant changes[24,25]. Thus the frequency of endoscopic surveillance of the retained rectum or ileal pouch to observe for dysplastic changes or malignancy must be considered.
Thus, for the reasons outlined above a considerable number of patients with a retained rectal stump who do not undergo reconstruction with IPAA or IRA will progress to require completion proctectomy. Traditionally, this would have been carried out via an open abdominoperineal approach. This however has been associated with significant morbidity (up to 41%) and mortality (6%), as well as long term perineal wound healing issues[22,23]. Advances in surgical practice in the form of laparoscopic[24] trans anal[25], robotic[26] and endoscopic[27] techniques have reduced this morbidity. Whether the anal canal is left-in situ or removed at time of proctectomy is an area of debate.
In this article, we aim to conduct a review of the published literature concerning the long-term management and surveillance of rectal stumps. As part of this, we hope to provide consolidated recommendations on surveillance guidelines for patients with rectal remnants following colectomy in ulcerative colitis. We also seek to provide a brief overview of medical management options of diversion proctitis, as well as a compendium of surgical methods of completion proctectomy in patients in whom medical management ultimately fails. As far as the authors are aware, there is currently no concise collection of this data in relation to the rectal stump available in the literature.
A systematic review was conducted according to the PRISMA guidelines[26]. Literature review of all papers providing recommendations for long term surveillance of patients with retained rectum following colectomy for ulcerative colitis with a retained rectum. The search was performed using multiple databases including MEDLINE (PubMed), Ovid and Cochrane databases including the timeframe between January 1980 and March 2020. Search protocol was cross checked in PROSPERO but no existing ongoing studies were found[27]. The following search criteria were used: Keywords (rectal) AND (stump) OR (remnant) OR (retained) AND (surveillance) AND (ulcerative colitis). Articles were included if they discussed recommendations on surveillance in patients with retained rectal tissue following colectomy for ulcerative colitis or indeterminate colitis. Papers were excluded if they dealt exclusively with Crohn’s colitis, paediatric populations and if recommendations made were specific to IPAA. Papers were also excluded if they made a general recommendation for surveillance, but gave no specific recommendation regarding timeframe or method of same.
A search and review of paper titles and abstracts was performed by the lead author, and papers were filtered to a list of those requiring full text evaluation. The references section of the papers undergoing evaluation were also examined for further relevant articles. The search was limited to English language studies. Data was extracted from each of the papers undergoing full text review including authors, year of publication, surveillance interval recommended and surveillance method. Descriptive statistics were used to analyse the data. Given the heterogeneity of the study populations and design, it was not possible to perform a meta-analysis. Results have been reported in line with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines.
Following this, a secondary search was carried out using the keywords (proctectomy), (rectal), (stump), (completion), (surgery), (ulcerative colitis) and (management). Again a search and review of paper titles and abstracts was performed by the lead author, and papers were filtered to a list of those requiring full text evaluation. The references section of the papers undergoing evaluation were also examined for further relevant articles. All articles reporting methods of completion rectal stump proctectomy were analysed.
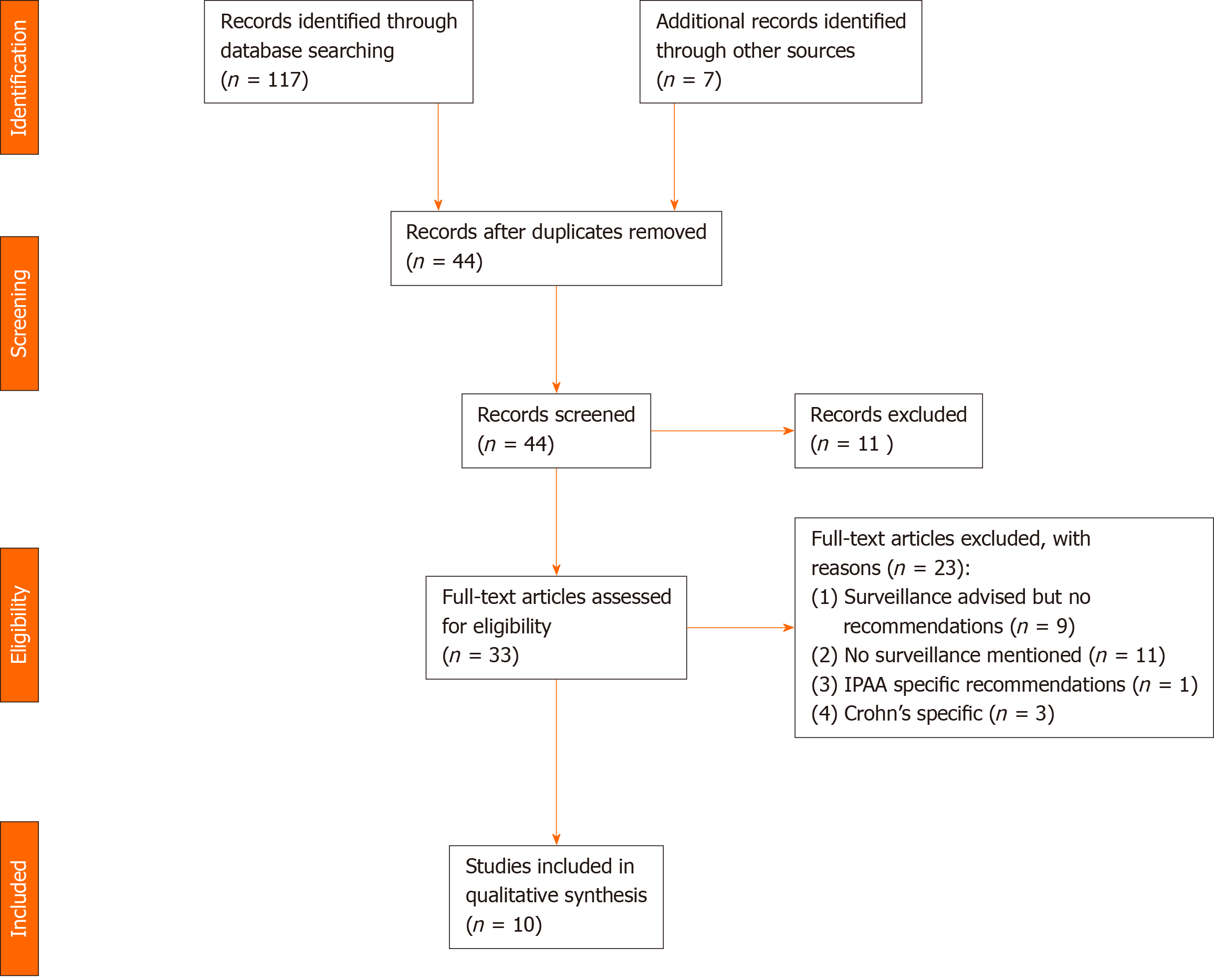
For the systematic review, initial search yielded 117 papers. After removal of duplicates and screening of abstracts, 37 papers were selected for further screening along with 7 additional papers found through review of references in the papers retrieved. Following analysis of each paper, 10 papers were included in the study. The reasons for exclusion are as per the criteria above and are outlined in the PRISMA flow diagram in Figure 1. Data was collected as above and the relevant papers and findings are summarised in Table 1[28-37]. Of the 10 papers, 2 (20%) recommended an interval of 6 mo to a year, 5 recommended yearly surveillance (50%), 1 (10%) recommended 2 yearly surveillance and the remaining 3 (30%) recommended risk stratification of patients and different screening intervals based on this. All studies agreed that follow up should be with endoscopy and biopsy, but there was no distinction made in terms of number or placement of biopsies. It is also worth noting that 9 further studies did highlight the importance of surveillance in this patients cohort, but made no specific recommendations regarding timing and/or method.
| Ref. | Year | Surveillance interval | Surveillance method |
| Pastore et al[28] | 1997 | Yearly | Endoscopy with biopsy |
| Khubchandani et al[29] | 1994 | 6 monthly to yearly | Endoscopy with biopsy |
| Petersen et al[30] | 2008 | 2 yearly | Endoscopy with biopsy |
| da Luz Moreira et al[31] | 2010 | Yearly | Endoscopy with biopsy |
| Lutgens et al[32] | 2012 | Rectal stump, PSC, and disease duration > 8 yr: 1 to 2 yearly | Endoscopy with biopsy |
| Munie et al[15] | 2013 | Yearly | Endoscopy with biopsy |
| Andersson et al[33] | 2014 | Yearly | Endoscopy with biopsy |
| Scoglio et al[34] | 2014 | 6 mo to yearly | Endoscopy with biopsy |
| Myrelid et al[36] | 2015 | < 20 yr of age + < 10 yr duration: yearly; early onset of the disease and > 10 yr duration: twice yearly; all others: yearly | Endoscopy with biopsy |
| Abdalla et al[35] | 2017 | Yearly | Endoscopy with biopsy |
| Derikx et al[37] | 2018 | High risk: yearly; Hx of PSC: 2-3 yearly; low risk: 5 yearly | Endoscopy with biopsy |
For the narrative aspect of the review, 253 articles were screened. Articles describing operative techniques for completion proctectomy in ulcerative colitis were assessed in full. Overall, a number of techniques were described in the literature including abdominoperineal resection, either open, laparoscopic or laparoscopic assisted, transanal approach either open or endoscopic, intersphincteric dissection and robotic proctectomy. These techniques will be explored in further detail within the discussion.
The long term risk of colorectal cancer (CRC) in patients with rectal remnants following colectomy in inflammatory bowel disease is well described[14,22]. In addition to the general risk of CRC associated with ulcerative colitis, patients post colectomy have the added factor of an “out of circuit,” rectum. This may mean that patients are less likely to be aware of signs of malignancy including changes in bowel habit or bleeding[15]. A high proportion of these patients may also suffer from diversion proctitis, which can present with discharge and bleeding and may mask signs of a more sinister pathology[20]. As a result these patients represent a more vulnerable population than those with intestinal continuity and pose a particular challenge for surveillance. In contrast, despite restored continuity, recent systematic review and meta-analysis by Derikx et al[22] also found the risk to be higher in those patients with IRA vs patients undergoing IPAA (2%-2.5% vs 5%). This is likely due to the higher proportion of retained rectal tissue in IRA and stump patients.
However, while recommendations exist for long term CRC surveillance in patients with inflammatory bowel disease[38-41], there is no specific guidance for patients with a retained rectal stump or IRA. The only guidelines to provide any form of recommendation for post-colectomy patients are the British Society of Gastroen-terology, however they do not distinguish between different operative interventions and the presence or absence of rectal remnants[42]. These guidelines stratify patients into low and high risk dependent on factors such as previous CRC history, dysplasia or history of primary sclerosing cholangitis (PSC). High risk patients are recommended for yearly screening and lower risk 5 yearly.
In in our literature review, many of the studies recommend between 6 moly and 2 yearly screening for patients with significant amounts of retained rectal tissue. More recent studies however have shifted focus toward risk stratification of patients based on disease duration and activity, history of previous CRC or dysplasia and disease related factors such as PSC. In 2015 Myrelid et al[36] advised guidelines based on patient disease duration. Based on analysis of the BSG guidelines, as well as guidelines in place for patients with an intact colon, Derikx et al[37] propose their own guidelines based on risk stratification into low intermediate and high risk groups. High risk cohorts with a history of CRC or dysplasia were advised to undergo yearly screening, intermediate cohorts with a history of PSC were advised 2-3 yearly screening and low risk patients 5 yearly[37]. In light of increased current knowledge regarding CRC risk in this patient cohort, this risk stratification strategy seems to be an appropriate method for providing guidance on surveillance. One major advantage of stratifying patients based on risk, is that it may reduce the number of follow up endoscopies needed overall in this patient group. This is an important consideration given that rectal stump patients are at a theoretical risk of stump blowout which each endoscopic procedure undertaken[43]. There is also a well-documented issue of overall compliance with endoscopic screening in this patient cohort[15,43,44] in particular with yearly follow up, and increasing the interval between scopes may improve compliance.
As introduced above, when discussing endoscopic surveillance it is also pertinent to discuss the risk of stump blowout[10]. Perforation or dehiscence of the rectal stump may occur following the initial colectomy or secondary to endoscopic surveillance[45]. Over vigorous endoscopy may result in perforation due to an increase in intraluminal pressure or direct scope trauma. Thus all endoscopists must be aware of this potential risk when performing surveillance of the rectal stump, particularly given the coexisting inflammation that is often present, and the endoscopist must be aware of the amount of rectum left in situ.
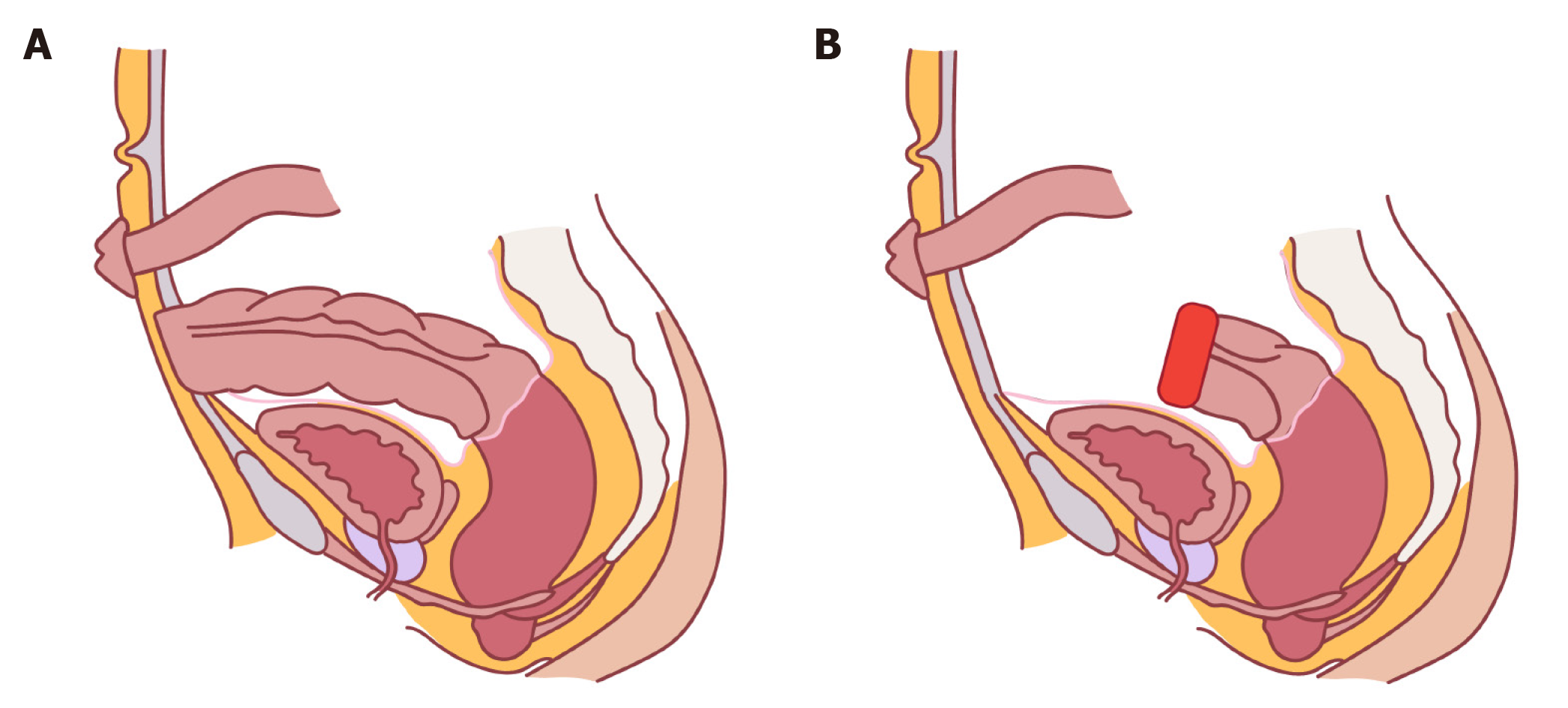
Traditionally the rectal stump was placed above the lower abdominal wall fascia. In this case, ensuing perforation/dehiscence which tends to occur at the apex of the staple line would result in a superficial wound infection. However, with the advent of minimally invasive surgery the trend now is to staple the remnant rectum above the peritoneal reflection intraperitoneally (Figure 2). However in this scenario any breakdown of the staple line may result in significant intra-abdominal or pelvic sepsis with systemic manifestations, carrying a risk of morbidity and mortality, prolonged hospital stay and the potential need for an emergent surgery. Severe non-resolving pain post endoscopy should alert the operator to the potential for perforation. An erect chest X-ray or computed tomography (CT) scan will show free air.
Clinically these patients may be difficult to manage. In the authors' experience this patient cohort may get quite sick despite having an end ileostomy in place. We hypothesize that the remnant rectum has a significant bacterial load which contaminates the sterile pelvic field post perforation. If the patient has ongoing infection that is not responding to conservative management then a CT abdomen and pelvis is indicated. If scanning shows a well-defined pelvic abscess then this may be drained percutaneously under radiological guidance. A tubogram will often show communication with the rectum. If radiological drainage is not feasible then a laparotomy may be required. If the patient has a long rectal stump then a staple line may be placed distal to the perforation site to healthy tissue. If there is only a short rectal stump that cannot be closed then an irrigation system may be used where a tube is placed proximally and via the anal canal and irrigation continued for 5-7 d[46].
Following colectomy, a number of patients may develop diversion proctitis or recurrent ulcerative proctitis in the retained rectum. In fact, endoscopic studies have shown that nearly all patients will go on to develop some level of inflammation, though less than 50% of these will be symptomatic[47-50]. For these patients, symptoms may include cramping abdominal pain, mucous or bloody discharge and tenesmus or anorectal pain[51]. In patients requiring treatment, nonsurgical management options include the use of short chain fatty acids (SCFA), topical 5-ASAs (American Society of Anesthesiologists), and topical glucocorticoids delivered via enema[21]. Despite early evidence for the efficacy of SCFA[52], more recent studies have doubted their efficacy[53,54], though butyrate enemas in particular may have an impact on tissue recovery[55] they are not widely used or available in practice. Topical 5-ASAs and steroids have also exhibited varying efficacy[56-60]. More experimental methods of management include fibre irrigation[61], endoscopic dextrose spray[62], leukocytapher-esis[63] and faecal transplantation[64], however the evidence for each of these methods is based on limited studies and case reports. If severe proctitis in a diverted rectum was refractory to 5ASA and topical steroids, consideration could be given to the addition of immunomodulators or biologics. However, the efficacy of medical management is highly variable and a proportion of patients will progress to requiring surgical input[21].
The authors generally favour removal of the anal canal at time of proctectomy, however it must be individualized to patient factors. Exceptions may include a patient with high ASA grades in whom reduced operative time is important, patients with associated fistulizing disease, hidradenitis suppurativa or other co-morbidities in which an anusectomy may be considered at a later stage when patient factors are more favourable. An early multidisciplinary approach in patients potentially needing surgery for ulcerative colitis is critical. In older patients, those with comorbidities or those who have a good understanding of the options who are not interested in reconstruction (IPAA) then the operating surgeon may consider a total proctocolectomy with end ileostomy as a single stage procedure, and avoid the need for a second intervention at a later stage. If clinically appropriate and the surgeon has the skill set, the entire procedure may be performed using a minimally invasive technique with the specimen removed via the perineum. However, in the emergency setting the majority of patients undergo a STC with end ileostomy. In the cohort of patients who elect not to undergo reconstruction with IPAA or IRA, a proportion (7%-14%) will eventually require completion proctectomy either as a result of ongoing proctitis or due to the development of dysplasia or CRC[65-67]. In these patients there are a number of options available in terms of surgical excision of the remaining rectum. While there have been no large scale, randomised trials encompassing all of these methods, there is adequate individual evidence supporting each one, and which method is employed often comes down to the operating surgeons training and preference.
The traditional gold standard approach would have consisted of an open abdominoperineal approach in the lithotomy position, with the perineal portion completed from below. The small bowel must be extruded from the pelvic field before starting proctectomy. Most surgeons follow the TME planes. Intramesorectal dissection has not been shown to reduce the nerve injury rate and is associated with increased blood loss. However this procedure is known to be associated with significant morbidity (41%) and mortality (up to 6%) of its own, as well as issues with perineal wound healing[65,68,69]. Further surgical advancement in this area has led to the evolution of hybrid approaches involving the use of laparoscopic, hand assisted laparoscopic/laparoscopic assisted surgery and robotic procedures for the abdominal component of dissection where required. Though recent studies in the use of laparoscopic surgery in ulcerative colitis for restorative proctocolectomy have found no major evidence for significant benefit of laparoscopic techniques[70,71], laparoscopic studies for proctectomy in oncology patients have indicated potential for shorter inpatient stays as well as faster return of bowel function[72] and there is also encouraging evidence for laparoscopic proctocolectomy and end ileostomy in ulcerative colitis patients[73,74]. There is also emerging evidence on the use of robotic technology, although this is still under investigation and there are issues surrounding accessibility for many institutions[73,75,76]. Irrespective of technique used completion proctectomy should be undertaken by a colorectal surgeon with considerable expertise given the associated morbidity.
An exclusively perineal approach can also be used, involving intersphincteric dissection from either a lithotomy or a prone, jack knife position. While not a commonly described approach for completion proctectomy in this patient cohort, in studies investigating the use of a jack knife approach for low rectal cancer it was associated with a significant reduction in operative complications including perineal infection and wound dehiscence as well as pelvic sepsis when compared to traditional lithotomy position[77,78]. Unlike an abdominoperineal resection for cancer there is no need for wide margins/extralevator dissection. We perform our dissection in the intersphincteric plane removing the internal sphincter complex and retaining the external sphincter complex. This reduces the size of the perineal defect reducing the potential for perineal wound breakdown and perineal herniation. Intersphincteric dissection may also have the benefit of reduced risk of impact on sexual function, as well as improved perineal healing. One of the draw-backs of this technique however is that it is difficult to employ in patients with longer rectal stumps[65], and may be technically more difficult in patients with significant adhesions[68,79,80].
Other, newer approaches are still being explored. This includes options such as transanal endoscopic microsurgery (TEMS)[81]. This is a relatively new technique for completion proctectomy, which may be considered in patients with longer rectal stumps which are not amenable to intersphincteric dissection. TEMS was initially described in the 80’s and involves the use of 10-20 cm proctoscopes that include a camera, suction and insufflation as well as ports for dissecting instruments. Generally it has been shown to be associated with less morbidity and shorter inpatient stays than open techniques[65]. In a small case series, Liyanage et al[81] describe the use of the technique in a cohort of 12 patients, nine of whom were undergoing the procedure due to IBD related complications. They found the technique to be particularly useful in those patients presenting with longer stumps and a potentially hostile abdomen, and overall found it to be both safe and effective. It is worth noting however that this is a highly specialised technique which requires extensive and specific training.
In conclusion, there is substantial evidence that patients with retained rectal tissue following STC have ongoing symptoms that interfere with their quality of life. In addition they require long term surveillance for dysplasia and CRC. While there are no clear guidelines produced by any governing body to guide surveillance intervals, stratification of patients into risk categories and basing intervals on this may be an appropriate solution for guiding long term follow up in this patient cohort. Great care must be taken when performing endoscopic surveillance and biopsies to avoid rectal stump perforation and ensuing pelvic sepsis. Patients with proctitis whose symptoms fail to respond to medical management may require completion proctectomy. A number of options are available for this, and again no strong evidence exists for one method over another. Similar to screening guidelines, in an era in which more of a focus is being placed on patient centred care and biopsychosocial models of health, the operating surgeon may have to decide on a case by case basis which intervention may be most appropriate, taking into account the patients co-morbidities, disease status, functional status and other factors such as age and lifestyle. When completion proctectomy is indicated the authors favour removal of the anal canal using intersphincteric dissection to reduce the potential for perineal wound breakdown or herniation.
Retained rectal stumps in ulcerative colitis carry long term risks including malignancy and recurrent disease. No clear body of literature exists on their long term management and surveillance.
To explore the current literature and provide a concise overview of the current evidence, as well as recommendations.
To provide an overview of options for the surgical management of remnant rectum and anal canal.
Systematic and narrative review of the literature.
All studies agreed surveillance should be carried out via endoscopy and biopsy. Increased vigilance is needed in endoscopy in these patients. Literature review revealed a number of options for surgical management of the remnant rectum.
Surveillance is necessary and should be risk stratified. The Authors favor intersphincteric dissection for removal of the rectal stump.
This is an important issue which requires ongoing research.
The authors would like to acknowledge Hennessy É for her contribution in the form of the illustrations in Figure 2.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Ireland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Choi YS, Yoon YS S-Editor: Gao CC L-Editor: A P-Editor: Li JH
| 1. | Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis. 2012;18:1356-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Bedrikovetski S, Dudi-Venkata N, Kroon HM, Liu J, Andrews JM, Lewis M, Lawrence M, Sammour T. Systematic review of rectal stump management during and after emergency total colectomy for acute severe ulcerative colitis. ANZ J Surg. 2019;89:1556-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1542] [Cited by in F6Publishing: 1895] [Article Influence: 270.7] [Reference Citation Analysis (1)] |
| 4. | Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol. 2015;50:942-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 228] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 5. | Mao EJ, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, Ananthakrishnan AN. Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther. 2017;45:3-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 6. | Kaplan GG, Seow CH, Ghosh S, Molodecky N, Rezaie A, Moran GW, Proulx MC, Hubbard J, MacLean A, Buie D, Panaccione R. Decreasing colectomy rates for ulcerative colitis: a population-based time trend study. Am J Gastroenterol. 2012;107:1879-1887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Vanga R, Long MD. Contemporary Management of Ulcerative Colitis. Curr Gastroenterol Rep. 2018;20:12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Targownik LE, Singh H, Nugent Z, Bernstein CN. The epidemiology of colectomy in ulcerative colitis: results from a population-based cohort. Am J Gastroenterol. 2012;107:1228-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 9. | Bernstein CN, Ng SC, Lakatos PL, Moum B, Loftus EV Jr; Epidemiology and Natural History Task Force of the International Organization of the Study of Inflammatory Bowel Disease. A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis. 2013;19:2001-2010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Carter MJ, Lobo AJ, Travis SP; IBD Section; British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 746] [Cited by in F6Publishing: 753] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 11. | Øresland T, Bemelman WA, Sampietro GM, Spinelli A, Windsor A, Ferrante M, Marteau P, Zmora O, Kotze PG, Espin-Basany E, Tiret E, Sica G, Panis Y, Faerden AE, Biancone L, Angriman I, Serclova Z, de Buck van Overstraeten A, Gionchetti P, Stassen L, Warusavitarne J, Adamina M, Dignass A, Eliakim R, Magro F, D'Hoore A; European Crohn's and Colitis Organisation (ECCO). European evidence based consensus on surgery for ulcerative colitis. J Crohns Colitis. 2015;9:4-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 12. | Macdermott RP, Green JA. Refractory ulcerative colitis treatment. Gastroenterol Hepatol (N Y). 2007;3:64-69. [PubMed] [Cited in This Article: ] |
| 13. | Frizelle FA, Burt MJ. Review: the surgical management of ulcerative colitis. J Gastroenterol Hepatol. 1997;12:670-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Ten Hove JR, Bogaerts JMK, Bak MTJ, Laclé MM, Meij V, Derikx LAAP, Hoentjen F, Mahmmod N, van Tuyl SA, Oldenburg B. Malignant and Nonmalignant Complications of the Rectal Stump in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:377-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Munie S, Hyman N, Osler T. Fate of the rectal stump after subtotal colectomy for ulcerative colitis in the era of ileal pouch-anal anastomosis. JAMA Surg. 2013;148:408-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Nordenvall C, Myrelid P, Ekbom A, Bottai M, Smedby KE, Olén O, Nilsson PJ. Probability, rate and timing of reconstructive surgery following colectomy for inflammatory bowel disease in Sweden: a population-based cohort study. Colorectal Dis. 2015;17:882-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Worley G, Nordenvall C, Askari A, Pinkney T, Burns E, Akbar A, Olén O, Ekbom A, Bottai M, Myrelid P, Faiz O. Restorative surgery after colectomy for ulcerative colitis in England and Sweden: observations from a comparison of nationwide cohorts. Colorectal Dis. 2018;20:804-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | O'Riordain DS, O'Connell PR. Completion proctectomy in ulcerative colitis. Br J Surg. 1997;84:436-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Wu XR, Liu XL, Katz S, Shen B. Pathogenesis, diagnosis, and management of ulcerative proctitis, chronic radiation proctopathy, and diversion proctitis. Inflamm Bowel Dis. 2015;21:703-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Kabir SI, Kabir SA, Richards R, Ahmed J, MacFie J. Pathophysiology, clinical presentation and management of diversion colitis: a review of current literature. Int J Surg. 2014;12:1088-1092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Tominaga K, Kamimura K, Takahashi K, Yokoyama J, Yamagiwa S, Terai S. Diversion colitis and pouchitis: A mini-review. World J Gastroenterol. 2018;24:1734-1747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 50] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Derikx LAAP, Nissen LHC, Smits LJT, Shen B, Hoentjen F. Risk of Neoplasia After Colectomy in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2016; 14: 798-806. e20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 23. | Lovegrove RE, Constantinides VA, Heriot AG, Athanasiou T, Darzi A, Remzi FH, Nicholls RJ, Fazio VW, Tekkis PP. A comparison of hand-sewn versus stapled ileal pouch anal anastomosis (IPAA) following proctocolectomy: a meta-analysis of 4183 patients. Ann Surg. 2006;244:18-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 24. | Shen B. Diagnosis and management of postoperative ileal pouch disorders. Clin Colon Rectal Surg. 2010;23:259-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Shen B, Lian L, Remzi FH, Bennet AE, Kiran PR, Queener EY, Lavery I, Fazio VW. T1294 Natural History and Outcome of Cuffitis in Ulcerative Colitis (UC) Patients With Restorative Proctocolectomy and Ileal Pouch-Anal Anastomosis (IPAA). AGA Abstracts. 2010;138:S-530. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47017] [Cited by in F6Publishing: 43253] [Article Influence: 2883.5] [Reference Citation Analysis (0)] |
| 27. | Siddaway AP, Wood AM, Hedges LV. How to Do a Systematic Review: A Best Practice Guide for Conducting and Reporting Narrative Reviews, Meta-Analyses, and Meta-Syntheses. Annu Rev Psychol. 2019;70:747-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 897] [Cited by in F6Publishing: 411] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 28. | Pastore RL, Wolff BG, Hodge D. Total abdominal colectomy and ileorectal anastomosis for inflammatory bowel disease. Dis Colon Rectum. 1997;40:1455-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 78] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Khubchandani IT, Kontostolis SB. Outcome of ileorectal anastomosis in an inflammatory bowel disease surgery experience of three decades. Arch Surg. 1994;129:866-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Petersen CN, Raahave D. [Adenocarcinoma in a closed rectal stump in inflammatory bowel disease]. Ugeskr Laeger. 2008;170:3251. [PubMed] [Cited in This Article: ] |
| 31. | da Luz Moreira A, Kiran RP, Lavery I. Clinical outcomes of ileorectal anastomosis for ulcerative colitis. Br J Surg. 2010;97:65-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Lutgens MW, van Oijen MG, Vleggaar FP, Siersema PD, Broekman MM, Oldenburg B; Dutch Initiative on Crohn and Colitis. Risk factors for rectal stump cancer in inflammatory bowel disease. Dis Colon Rectum. 2012;55:191-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Andersson P, Norblad R, Söderholm JD, Myrelid P. Ileorectal anastomosis in comparison with ileal pouch anal anastomosis in reconstructive surgery for ulcerative colitis--a single institution experience. J Crohns Colitis. 2014;8:582-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Scoglio D, Ahmed Ali U, Fichera A. Surgical treatment of ulcerative colitis: ileorectal vs ileal pouch-anal anastomosis. World J Gastroenterol. 2014;20:13211-13218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 40] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Abdalla M, Landerholm K, Andersson P, Andersson RE, Myrelid P. Risk of Rectal Cancer After Colectomy for Patients With Ulcerative Colitis: A National Cohort Study. Clin Gastroenterol Hepatol 2017; 15: 1055-1060. e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Myrelid P, Øresland T. A reappraisal of the ileo-rectal anastomosis in ulcerative colitis. J Crohns Colitis. 2015;9:433-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Derikx LAAP, de Jong ME, Hoentjen F. Short article: Recommendations on rectal surveillance for colorectal cancer after subtotal colectomy in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2018;30:843-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 2010; 138: 746-774, 774. quiz e12-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 316] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 39. | Annese V, Beaugerie L, Egan L, Biancone L, Bolling C, Brandts C, Dierickx D, Dummer R, Fiorino G, Gornet JM, Higgins P, Katsanos KH, Nissen L, Pellino G, Rogler G, Scaldaferri F, Szymanska E, Eliakim R; ECCO. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J Crohns Colitis. 2015;9:945-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 288] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 40. | National Institute for Health and Care Excellence Clinical Guideline 118. Colonoscopic surveillance for prevention of colorectal cancer in people with ulcerative colitis, Crohn’s disease or adenomas. Available from: https://www.nice.org.uk/guidance/cg118/evidence/full-guideline-pdf-181410157. [Cited in This Article: ] |
| 41. | American Society for Gastrointestinal Endoscopy Standards of Practice Committee, Shergill AK, Lightdale JR, Bruining DH, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Foley K, Hwang JH, Jue TL, Khashab MA, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf R, Cash BD, DeWitt JM. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc 2015; 81: 1101-21. Gastrointest Endosc. 2015;81:1101-21.e1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 42. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP, Lucassen A, Jenkins P, Fairclough PD, Woodhouse CR; British Society of Gastroenterology; Association of Coloproctology for Great Britain and Ireland. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 819] [Cited by in F6Publishing: 769] [Article Influence: 54.9] [Reference Citation Analysis (2)] |
| 43. | Landerholm K, Wood C, Bloemendaal A, Buchs N, George B, Guy R. The rectal remnant after total colectomy for colitis - intra-operative,post-operative and longer-term considerations. Scand J Gastroenterol. 2018;53:1443-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Juviler A, Hyman N. Ulcerative colitis: the fate of the retained rectum. Clin Colon Rectal Surg. 2004;17:29-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Arnell TD. Surgical management of acute colitis and toxic megacolon. Clin Colon Rectal Surg. 2004;17:71-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Johnston S, De Lacavalerie P. Management of rectal stump leak following emergency Hartmann’s procedure. J Coloproctology. 2020;40:386-389. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Korelitz BI, Cheskin LJ, Sohn N, Sommers SC. The fate of the rectal segment after diversion of the fecal stream in Crohn's disease: its implications for surgical management. J Clin Gastroenterol. 1985;7:37-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Glotzer DJ, Glick ME, Goldman H. Proctitis and colitis following diversion of the fecal stream. Gastroenterology. 1981;80:438-441. [PubMed] [Cited in This Article: ] |
| 49. | Haque S, Eisen RN, West AB. The morphologic features of diversion colitis: studies of a pediatric population with no other disease of the intestinal mucosa. Hum Pathol. 1993;24:211-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Geraghty JM, Talbot IC. Diversion colitis: histological features in the colon and rectum after defunctioning colostomy. Gut. 1991;32:1020-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 74] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Ma CK, Gottlieb C, Haas PA. Diversion colitis: a clinicopathologic study of 21 cases. Hum Pathol. 1990;21:429-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989;320:23-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 596] [Cited by in F6Publishing: 508] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 53. | Pal K, Tinalal S, Al Buainain H, Singh VP. Diversion proctocolitis and response to treatment with short-chain fatty acids--a clinicopathological study in children. Indian J Gastroenterol. 2015;34:292-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Schauber J, Bark T, Jaramillo E, Katouli M, Sandstedt B, Svenberg T. Local short-chain fatty acids supplementation without beneficial effect on inflammation in excluded rectum. Scand J Gastroenterol. 2000;35:184-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Luceri C, Femia AP, Fazi M, Di Martino C, Zolfanelli F, Dolara P, Tonelli F. Effect of butyrate enemas on gene expression profiles and endoscopic/histopathological scores of diverted colorectal mucosa: A randomized trial. Dig Liver Dis. 2016;48:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 56. | Triantafillidis JK, Nicolakis D, Mountaneas G, Pomonis E. Treatment of diversion colitis with 5-aminosalicylic acid enemas: comparison with betamethasone enemas. Am J Gastroenterol. 1991;86:1552-1553. [PubMed] [Cited in This Article: ] |
| 57. | Tripodi J, Gorcey S, Burakoff R. A case of diversion colitis treated with 5-aminosalicylic acid enemas. Am J Gastroenterol. 1992;87:645-647. [PubMed] [Cited in This Article: ] |
| 58. | Grisham MB, Granger DN. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988;33:6S-15S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 276] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 59. | Jowett SL, Cobden I. Diversion colitis as a trigger for ulcerative colitis. Gut. 2000;46:294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Lim AG, Langmead FL, Feakins RM, Rampton DS. Diversion colitis: a trigger for ulcerative colitis in the in-stream colon? Gut. 1999;44:279-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | de Oliveira-Neto JP, de Aguilar-Nascimento JE. Intraluminal irrigation with fibers improves mucosal inflammation and atrophy in diversion colitis. Nutrition. 2004;20:197-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Nyabanga CT, Shen B. Endoscopic Treatment of Bleeding Diversion Pouchitis with High-Concentration Dextrose Spray. ACG Case Rep J. 2017;4:e51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Watanabe C, Hokari R, Miura S. Chronic antibiotic-refractory diversion pouchitis successfully treated with leukocyteapheresis. Ther Apher Dial. 2014;18:644-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Gundling F, Tiller M, Agha A, Schepp W, Iesalnieks I. Successful autologous fecal transplantation for chronic diversion colitis. Tech Coloproctol. 2015;19:51-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Middleton PF, Sutherland LM, Maddern GJ. Transanal endoscopic microsurgery: a systematic review. Dis Colon Rectum. 2005;48:270-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 66. | Böhm G, O'Dwyer ST. The fate of the rectal stump after subtotal colectomy for ulcerative colitis. Int J Colorectal Dis. 2007;22:277-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Brady RR, Collie MH, Ho GT, Bartolo DC, Wilson RG, Dunlop MG. Outcomes of the rectal remnant following colectomy for ulcerative colitis. Colorectal Dis. 2008;10:144-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Lubbers EJ. Healing of the perineal wound after proctectomy for nonmalignant conditions. Dis Colon Rectum. 1982;25:351-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Genua JC, Vivas DA. Management of nonhealing perineal wounds. Clin Colon Rectal Surg. 2007;20:322-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Singh P, Bhangu A, Nicholls RJ, Tekkis P. A systematic review and meta-analysis of laparoscopic vs open restorative proctocolectomy. Colorectal Dis. 2013;15:e340-e351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Neumann PA, Rijcken E. Minimally invasive surgery for inflammatory bowel disease: Review of current developments and future perspectives. World J Gastrointest Pharmacol Ther. 2016;7:217-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Khaikin M, Bashankaev B, Person B, Cera S, Sands D, Weiss E, Nogueras J, Vernava A 3rd, Wexner SD. Laparoscopic versus open proctectomy for rectal cancer: patients' outcome and oncologic adequacy. Surg Laparosc Endosc Percutan Tech. 2009;19:118-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 73. | Holder-Murray J, Marsicovetere P, Holubar SD. Minimally invasive surgery for inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1443-1458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Holder-Murray J, Zoccali M, Hurst RD, Umanskiy K, Rubin M, Fichera A. Totally laparoscopic total proctocolectomy: a safe alternative to open surgery in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:863-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Miller AT, Berian JR, Rubin M, Hurst RD, Fichera A, Umanskiy K. Robotic-assisted proctectomy for inflammatory bowel disease: a case-matched comparison of laparoscopic and robotic technique. J Gastrointest Surg. 2012;16:587-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 76. | Marino MV, Glagoleva A. P429 Robotic-assisted vs. laparoscopic proctectomy for inflammatory bowel disease: Results of the case-match comparison in single institution. J Crohns Colitis. 2018;12:S322-S322. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Liu P, Bao H, Zhang X, Zhang J, Ma L, Wang Y, Li C, Wang Z, Gong P. Better operative outcomes achieved with the prone jackknife vs. lithotomy position during abdominoperineal resection in patients with low rectal cancer. World J Surg Oncol. 2015;13:39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Showalter SL, Kelz RR, Mahmoud NN. Effect of technique on postoperative perineal wound infections in abdominoperineal resection. Am J Surg. 2013;206:80-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Leicester RJ, Ritchie JK, Wadsworth J, Thomson JP, Hawley PR. Sexual function and perineal wound healing after intersphincteric excision of the rectum for inflammatory bowel disease. Dis Colon Rectum. 1984;27:244-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Lyttle JA, Parks AG. Intersphincteric excision of the rectum. Br J Surg. 1977;64:413-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 89] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Liyanage C, Ramwell A, Harris GJ, Levy BF, Simson JN. Transanal endoscopic microsurgery: a new technique for completion proctectomy. Colorectal Dis. 2013;15:e542-e547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |