Published online Nov 30, 2009. doi: 10.4240/wjgs.v1.i1.49
Revised: November 12, 2009
Accepted: November 19, 2009
Published online: November 30, 2009
AIM: To elucidate the influence of liver cirrhosis (LC) on the prognosis of patients with gastric cancer (GC).
METHODS: Of the 1347 GC patients who underwent curative gastrectomy for GC between January 1984 and June 2007, 25 patients (21 men and 4 women with a median age of 67 years; range 54-77 years) with LC were enrolled in this study. Using the Child-Pugh classification, 15 patients were evaluated as grade A and 10 patients as grade B. No grade C patient underwent gastrectomy in this series. Clinical outcomes, including postoperative morbidity and survival, were retrospectively analyzed based on medical records and surgical files.
RESULTS: There was no significant difference in operative blood loss and perioperative blood transfusion between the two groups. The most common postoperative complication was intractable ascites, which was the single postoperative morbidity noted more frequently in grade B patients (40.0%) than in grade A patients (6.7%) with statistical significance (P = 0.041). Operative mortality due to hepatic failure was seen in one grade A patient. Three patients had hepatocellular carcinoma (HCC) at presentation and two patients developed HCC after surgery. Overall 5-year survival rate was 58.9% in patients with early GC and 33.3% in patients with advanced GC (P = 0.230). GC-specific 5-year survival rate of early GC patients was 90.0% while that of advanced GC patients was 58.3% (P = 0.010). Four patients with early GC died of uncontrolled HCC, of which two were synchronous and two metachronous.
CONCLUSION: The risk of postoperative intractable ascites is high, particularly in grade B patients. Early detection and complete control of HCC is vital to improve a patient’s prognosis.
- Citation: Ikeda Y, Kanda T, Kosugi SI, Yajima K, Matsuki A, Suzuki T, Hatakeyama K. Gastric cancer surgery for patients with liver cirrhosis. World J Gastrointest Surg 2009; 1(1): 49-55
- URL: https://www.wjgnet.com/1948-9366/full/v1/i1/49.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v1.i1.49
Two clinical problems should be considered in the management of gastric cancer (GC) patients with liver cirrhosis (LC): safety of the surgery and patient prognosis, which is potentially worsened by a LC-related death in the long term.
It is well known that patients with LC have a high incidence of postoperative complications and postoperative mortality[1-4]. Several studies assessing the risk of GC surgery in LC-complicated patients have been reported and the usefulness of the Child classification has been noted[5,6]. However, although the Child classification is convenient and widely used, its lack of objectivity is a cause for concern. Recently, the Child-Pugh classification[7], which replaces assessment factors with laboratory data and scores them, is more frequently used to evaluate overall hepatic function. However, it remains uncertain whether or not this modified classification is sufficiently reliable to assess the risk of GC surgery as only a limited number of studies have been conducted.
With regard to the prognosis of patients with GC associated with LC, Isozaki et al[6] reported that the 5-year survival rate after gastrectomy is 64% in patients with early GC and 14% in those with advanced GC. In a recent multi-center, retrospective study on patients with GC associated with LC conducted in South Korea[8], the 5-year survival rate was 73% in patients with early GC and 26% in those with advanced GC. These studies have shown that the prognosis is poorer in patients with GC associated with LC than in GC patients with no complications. Early studies have shown that LC-related deaths, including hepatic failure, esophagogastric bleeding and liver cancer, may have lowered overall patient survival. However, data on the incidence, treatment and outcome of LC-related diseases or the impact on patient survival are lacking. Clearly, the prognosis of GC patients with LC is affected not only by GC but also by LC and synchronous or metachronous hepatocellular carcinoma (HCC). Therefore, to determine the therapeutic strategy for GC associated with LC, it is necessary to know precisely the effects of LC-related diseases on the prognosis of patients after GC surgery.
In this study, the usefulness of risk assessment of GC surgery based on the currently used Child-Pugh classification was evaluated. In addition, by investigating in detail the prognosis of patients, the effects of LC and LC-related diseases on the long-term prognosis of patients with GC are shown.
A total of 1347 consecutive patients underwent a gastrectomy for GC at Niigata University Medical and Dental Hospital between January 1984 and June 2007. Of the 1347 GC patients, 25 (1.9%) were diagnosed as having LC and these 25 patients were enrolled in this study. The diagnosis of LC was made on the basis of clinical findings and laboratory tests. Clinical findings included preoperative imaging and intraoperative findings. The presence or absence of ascites, splenomegaly, irregularity of liver surface, atrophy of the right lobe, and hypertrophy of the left lobe were evaluated by abdominal ultrasonography and computed tomography. Diffuse scarring of the liver, characterized by loss of lobular architecture and the formation of regenerative nodules, was a typical finding. Intraoperative liver biopsy performed in 4 of the 25 patients confirmed the histological diagnosis of LC based on the presence of fibrous septa and regenerative nodules. The patients were comprised of 21 men (84.0%) and 4 women (16.0%) with a median age of 67 years (range 54-77 years). Of the 25 GC patients with LC in this study, 15 patients were evaluated as grade A and 10 as grade B using the Child-Pugh classification. There were no grade C patients who underwent a gastrectomy in this series. The median follow-up time was 45 mo (range 6-168 mo).
To assess the severity of liver damage, the Child-Pugh classification was used. Laboratory tests included serologic tests for hepatitis B virus surface antigen (HBV) and hepatitis C virus antibody (HCV), hematochemical tests, and coagulation tests for grading using the Child-Pugh classification. Indocyanine green (ICG) disappearance rate was determined and used as an indicator of hepatic functional reserve in 20 patients. After intravenous injection of ICG (0.5 mg/kg), plasma ICG disappearance rate (K-ICG) was calculated by linear regression from plasma ICG concentrations at 5, 10 and 15 min.
The description of GC, including primary lesions, metastatic lesions, staging, surgical treatment and histological findings, was recorded in accordance with the Japanese Classification of Gastric Carcinoma[9]. Early cancer was defined as a T1 tumor while advanced cancer was defined as ≥ a T2 tumor, regardless of nodal involvement.
A total or distal gastrectomy was selected depending on the location and the macroscopic type of GC. When the tumor was located in the middle or lower third of the stomach, a distal gastrectomy was performed. When the cancer-free margin could not be ensured by a distal gastrectomy due to an infiltrative growth pattern, a total gastrectomy was selected regardless of the main location of the tumor. A modified gastrectomy A was defined as a gastrectomy with lymph node dissection of all Group 1 nodes in addition to lymph nodes along the left gastric artery according to GC treatment guidelines[10]. A wedge resection was performed when a patient had early GC and limited hepatic functional reserve (ICG retention rate at 15 min > 40%), or when it was necessary to perform a portosystemic shunt operation simultaneously due to serious esophageal varices.
Postoperative morbidity included intractable ascites (abdominocentesis and/or increased diuretics required), hepatic failure [clinical manifestations of edema, ascites, hyperbilirubinemia (defined as serum total bilirubin > 2.0 mg/dL), and ammonemia (defined as blood ammonia > 100 μg/dL)], intraabdominal infection (positive bacterial culture of drainage fluid), anastomotic leakage, and sepsis (proven by blood culture). Postoperative mortality was defined as any death occurring during the hospital stay for the gastrectomy.
Pearson’s χ2 test and Fisher’s exact test were used to analyze associations for the comparison of two factors. Overall survival was calculated from the time of gastrectomy until death from any cause or the most recent follow-up for surviving patients. GC-specific survival rate was calculated from the time of gastrectomy until death from GC. Survival curves were estimated using the Kaplan-Meier method and compared by means of the log-rank test. The Mann-Whitney test was used to compare continuous variables between groups. All statistical evaluations were performed using StatView version 5.0 software (SAS Institute, Cary, NC, USA). All tests were two-sided and values of P < 0.05 were considered statistically significant.
The results of preoperative blood studies, laboratory tests, and coagulation tests of the 25 patients are shown in Table 1. Significant decreases in hemoglobin, platelet count, serum albumin, and prothrombin time were found in the grade B group as compared to the grade A group. Total bilirubin, aspartate aminotransferase and hepatic functional reserve determined using ICG, were not significantly different between the two groups.
| Grade A (n = 15) | Grade B (n = 10) | P value | |
| Hemoglobin (g/dL) | 13.5 ± 2.0 | 10.2 ± 2.8 | 0.006 |
| Platelet (104/μL) | 13.0 ± 6.1 | 9.3 ± 9.6 | 0.040 |
| Albumin (g/dL) | 3.9 ± 0.5 | 3.0 ± 0.4 | 0.001 |
| Total bilirubin (mg/dL) | 0.8 ± 0.3 | 0.9 ± 0.5 | 0.696 |
| Aspartate aminotransferase (IU/L) | 40 ± 22 | 37 ± 15 | 0.824 |
| Prothrombin time (n = 24, %) | 90 ± 19 | 68 ± 18 | 0.017 |
| ICG R15 (n = 20, %) | 24.3 ± 15.0 | 30.6 ± 14.2 | 0.285 |
| K-ICG (n = 20) | 0.110 ± 0.033 | 0.092 ± 0.047 | 0.165 |
The clinicopathological characteristics of the 25 patients using the Child-Pugh classification are shown in Table 2. The most common cause of cirrhosis was alcohol in the grade A group and virus in the grade B group. The grade B group had significantly more patients with intraoperative ascites than the grade A group (P = 0.001). Distal gastrectomy, total gastrectomy, and wedge resection were performed in 12 (48.0%), 10 (40.0%), and 3 (12.0%) patients, respectively. Three patients underwent a total gastrectomy with splenectomy and a further 3 patients underwent a splenectomy and caudal pancreatectomy for complete lymph node dissection. A D1 dissection was the most common extent of lymph node dissection, most of which were conducted in combination with a modified gastrectomy A. None of the patients presented with a residual tumor macroscopically after gastrectomy. Sixteen tumors (64.0%) were histologically diagnosed as early cancer, while 9 (36.0%) were diagnosed as advanced cancer. Lymph node metastasis was microscopically found in 5 patients (20.0%). Depth of invasion, lymph node metastasis, extent of gastric resection, and that of lymph node dissection were not significantly different between the two groups.
| Grade A (n = 15) | Grade B (n = 10) | P value | |
| Cause of cirrhosis | |||
| Alcohol | 9 | 3 | 0.369 |
| Virus | 5 | 6 | |
| HCV-related | 5 | 4 | |
| HBV-related | 0 | 1 | |
| Both | 0 | 1 | |
| Unknown | 1 | 1 | |
| Preoperative esophageal varices | 5 | 7 | 0.111 |
| Intraoperative ascites | 0 | 6 | 0.001 |
| Extent of gastric resection | |||
| Distal | 7 | 5 | 0.517 |
| Total | 7 | 3 | |
| Wedge | 1 | 2 | |
| Extent of lymph node dissection | |||
| D0 | 2 | 3 | 0.627 |
| D1 (modified gastrectomy A) | 8 (6) | 4 (4) | |
| D2 | 4 | 3 | |
| D3 | 1 | 0 | |
| Residual tumor | |||
| R0 | 15 | 10 | > 0.999 |
| R1/2 | 0 | 0 | |
| Depth of invasion | |||
| pT1 | 9 | 7 | 0.407 |
| pT2 | 5 | 2 | |
| pT3 | 1 | 1 | |
| Lymph node metastasis | |||
| pN0 | 11 | 6 | 0.758 |
| pN1 | 1 | 1 | |
| pN2 | 1 | 1 | |
| pN3 | 1 | 0 | |
| pNX1 | 1 | 2 |
Table 3 compares operative blood loss, perioperative blood transfusion of packed red blood cells (PRBC) and fresh frozen plasma (FFP), and postoperative morbidity and mortality between the two groups. Operative blood loss and perioperative blood transfusion were not significantly different between the two groups although the mean amounts were larger in the grade B group. The most common postoperative complication was intractable ascites which was the only postoperative morbidity noted more frequently in grade B patients (40.0%) than in grade A patients (6.7%) with statistical significance. Postoperative hospital stay was not significantly different between the two groups.
| Grade A (n = 15) | Grade B (n = 10) | P value | |
| Mean operative blood loss (range, mL) | 754 (79-2705) | 1103 (182-3073) | 0.336 |
| Perioperative transfusion of PRBC | 5 (33.3) | 6 (60.0) | 0.241 |
| Mean volume of transfused PRBC (range, mL) | 208 (0-1040) | 416 (0-1040) | 0.198 |
| Perioperative transfusion of FFP | 12 (80.0) | 6 (60.0) | 0.378 |
| Mean volume of transfused FFP (range, mL) | 1904 (0-7760) | 2968 (0-10 000) | 0.575 |
| Morbidity | 7 (46.7) | 6 (60.0) | 0.688 |
| Ascites | 1 (6.7) | 4 (40.0) | 0.041 |
| Intraabdominal infection | 0 | 2 (20.0) | 0.071 |
| Hepatic failure | 3 (20.0) | 0 | 0.132 |
| Leakage | 2 (13.3) | 0 | 0.229 |
| Sepsis | 1 (6.7) | 0 | 0.405 |
| Mortality | 1 (6.7)1 | 0 | 0.405 |
| Median postoperative hospital stay (range, d) | 34 (12-63) | 29 (12-41) | 0.322 |
In this series, 20 patients underwent the ICG test to evaluate hepatic functional reserve. Eleven patients had K-ICGs higher than 0.1 and 9 had K-ICGs lower than 0.1. K-ICG was not associated with postoperative complications: there were 8 patients (72.7%) in the high K-ICG group and 2 (22.2%) in the low K-ICG group (P = 0.07). As regards intractable ascites, no significant difference was found between the two groups: there were 3 patients (27.2%) in the high K-ICG group and 1 patient (11.1%) in the low K-ICG group (P = 0.591).
Eight patients underwent a D2 or D3 lymph node dissection. Of the 8 patients, 3 (37.5%) had postoperative complications. However, in the 17 patients who underwent a D0 or D1 lymph node dissection, 10 (58.8%) had postoperative complications. No significant association was found between the extent of dissection and postoperative complications in this series (P = 0.411). For intractable ascites, no significant difference was found between the two groups: there was 1 patient (12.5%) in the D2 or D3 dissection group and 3 patients (27.2%) in the D0 or D1 dissection group (P > 0.999).
Of the 3 patients who developed hepatic failure, one died of multiple organ failure on postoperative day 11. This patient had undergone a total gastrectomy with splenectomy, caudal pancreatectomy, and lower esophagectomy for advanced GC with esophageal invasion. Severity of LC of the patient was grade A using the Child-Pugh classification, the K-ICG value was 0.090 and the ICG R15 rate was 29.3%.
Of the 25 patients, 12 patients had preoperative esophageal varices. Three of these 12 patients underwent a portosystemic shunt operation. Two underwent a left gastric vein - inferior vena cava shunt operation (Inokuchi shunt[11]) and one an inferior mesenteric- left renal vein shunt operation, in combination with a gastrectomy due to severe varices. In these 3 patients, varices were in remission or stable after surgery. Although the remaining 9 patients did not undergo a shunt operation simultaneously, one patient showed worsened varices and underwent a shunt operation 33 mo after gastrectomy.
There were 3 patients who had synchronous solitary HCC in this study. One grade A patient with a tumor 2.5 cm in diameter received intra- and postoperative percutaneous ethanol injection (PEI); one grade B patient with a tumor 1.5 cm in diameter underwent combined procedures, including intraoperative radiofrequency ablation (RFA), postoperative PEI, and transarterial embolization (TAE); and the other grade B patient with HCC 1.8 cm in diameter declined to undergo any treatment.
A total of 2 grade A patients developed metachronous solitary HCC after gastrectomy. The intervals between gastric cancer surgery and diagnosis of HCC in the 2 patients were 54 and 113 mo. One patient underwent PEI and TAE and the treatment for the other patient is unknown. The clinicopathological characteristics of the 5 patients with HCC are summarized in Table 4.
| Age (yr) | C-P | pT | pN | Surgery for gastric cancer | HCC | Treatment for HCC | Cause of death | Survival time (mo) |
| 71 | Grade A | pT1 | pNX | Wedge resection, D0 | SYN | PEI | HCC | 22 |
| 62 | Grade A | pT1 | pN0 | Distal GR, D2 | MET | PEI, TAE | HCC | 80 |
| 55 | Grade A | pT1 | pN0 | Distal GR, D1 | MET | Unknown | HCC | 121 |
| 68 | Grade B | pT1 | pN0 | Distal GR, D1 | SYN | RFA, PEI, TAE | Alive | 64 |
| 81 | Grade B | pT1 | pN0 | Distal GR, D1 | SYN | None | HCC | 46 |
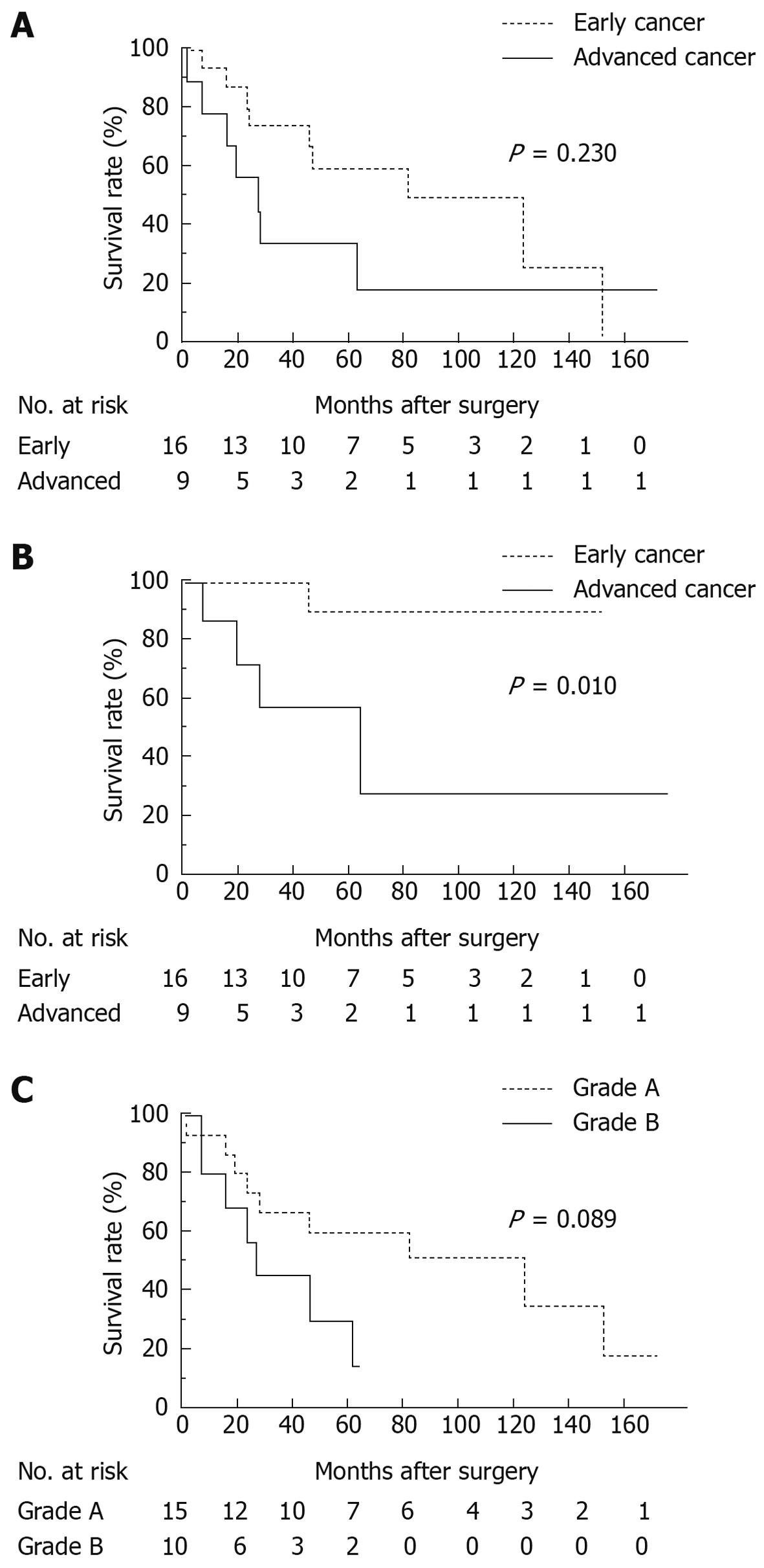
Overall 5-year survival rate for all patients was 49.6%: 58.9% for patients with early GC and 33.3% for patients with advanced GC. Median survival time was 53 and 26 mo respectively (Figure 1A, P = 0.230). GC-specific 5-year survival rate for patients with early GC was 90.0%, while that for patients with advanced GC was 58.3% (Figure 1B, P = 0.010). Comparing grades A and B using the Child-Pugh classification, overall 5-year survival rate for grade A patients was 60.0% and 30.5% for grade B patients with the median survival time of 59 and 23 mo respectively (Figure 1C, P = 0.089).
At the time of writing, 16 of the 25 patients died after GC surgery. The causes of death, excluding one postoperative mortality due to hepatic failure, were as follows: GC in 5 patients; HCC in 4; and 1 for each of the following: lung cancer, cerebral infarction, pneumonia, chronic heart failure, accident, and unknown cause. There were no deaths from chronic hepatic failure or fatal gastrointestinal hemorrhage due to ruptured esophagogastric varices.
The most common cause of death was GC recurrence in patients with advanced GC (4 of 7 deaths) and HCC in patients with early GC (4 of 9 deaths). All of the 4 deaths from HCC were in patients with early GC. Two patients who had synchronous solitary HCC died of HCC within 5 years after the gastrectomy (Table 4).
The assessment of perioperative risk of LC is a surgically important issue. In this study, GC patients with LC were divided into two groups according to the Child-Pugh classification and the perioperative outcome was analyzed. Of the 25 patients, 15 were classified as grade A and 10 as grade B. The one case of postoperative mortality in this series involved a patient with an advanced GC located in the upper and middle stomach who underwent total gastrectomy, splenectomy, and caudal pancreatectomy together with a D2 dissection including the dissection around the hepatoduodenal ligament. The patient developed postoperative hepatic failure and subsequently multiple organ failure, and died on postoperative day 11. The Child-Pugh score of this patient was 5 and LC was grade A. With respect to complications from surgery, the incidence was generally high: 46.7% in grade A patients and 60.0% in grade B patients. However, there was no significant statistical difference in these two groups and these results show the limitation of risk assessment of GC surgery using the Child-Pugh classification. Conversely, even for grade B patients, radical GC surgery can be performed safely if the surgeon selects the appropriate surgical procedure and prudent care is given to the patient postoperatively. Very recently, Jang et al[8] reported the surgical outcomes of 57 GC patients with LC who underwent a radical gastrectomy in 3 university hospitals in South Korea. The Child-Pugh classification system was used for the surgical risk assessment. Operative mortality was noted in only 2 (4.3%) of 46 GC patients with grade A cirrhosis, while it occurred in 3 (27.3%) of 11 GC patients with grade B/C cirrhosis, a significantly larger number than that of grade A patients. In addition, Isozaki et al[6] reported that 4 of 26 GC patients with grade B/C cirrhosis based on the Child classification developed serious complications after GC surgery, and 2 (8.0%) died of complications from renal failure and hepatic failure. The mortality rate of grade B cirrhosis patients who underwent GC surgery varies greatly by investigator, possibly because of the paucity of cases analyzed. Clinical studies on GC surgery of patients with grade B or higher LC are very few: there are only five studies[5,6,8,12] including this one, that have analyzed 10 patients or more. To obtain reliable data on operative mortality, an event with very low incidence, analysis of a large patient population based on a nationwide investigation is needed.
This study showed no significant difference in the incidence of overall postoperative complications between grade A and grade B GC patients. However, the development of postoperative intractable ascites was significantly more frequent in grade B patients. All earlier studies showed that, in GC surgery of patients with LC, the incidence of intractable ascites is high, which is clinically important in relation to the extent of lymph node dissection in GC. Lee et al[12] reported the surgical outcome of 94 GC patients with LC who underwent a gastrectomy with a D2 lymph node dissection. Two patients classified as grade B or C according to the Child classification died of postoperative complications from hepatorenal failure with intractable ascites. No serious complications or operative mortality was noted in 82 patients who were classified as grade A. In these patients, ascites was also the common postoperative complication but was well controlled by medication and/or paracentesis. Therefore, Lee et al[12] concluded that the presence of compensated cirrhosis, such as grade A, was not a contraindication to a gastrectomy with a D2 or more lymph node dissection. Ryu et al[13] reported the surgical outcome of 26 GC patients with LC classified as grade A using the Child-Pugh classification. They found no serious complications or operative mortality although 8 patients received diuretics and one required abdominocentesis. In their series, 25 of the 26 patients underwent a D2 lymph node dissection. Based on these findings, Ryu et al[13] claimed that a D2 lymph node dissection could be safely applied to GC patients with low-grade LC, such as a grade A. Jang et al[8] concluded that a D2 lymph node dissection in grade B patients should be avoided because they found that in patients undergoing a D2 dissection, none of the grade A patients had developed massive ascites postoperatively, while 3 of 4 grade B patients developed massive ascites. Also, Isozaki et al[6] reported 3 in-hospital deaths among 19 GC patients with LC who underwent D2 or more extensive dissection. Of note was that 2 of the 3 patients died of organ failure following the development of significant ascites that was caused by dissection around the hepatoduodenal ligament, as in the one case of death in this series. In this study, 4 of 5 patients who had developed intractable ascites were grade B patients: 3 patients underwent a D0 or D1 dissection; one patient underwent a D2 dissection. Collectively, with regard to the extent of lymph node dissection in LC patients, a D2 dissection should be avoided in grade B patients. In addition, the hepatoduodenal ligament should be kept intact as much as possible, even in grade A patients.
It is widely known that the prognosis of GC patients with LC is poor. Nevertheless, it is essential to have more detailed information on the degree and the cause to establish a better therapeutic strategy. This study showed that the overall 5-year survival rate of early GC patients with LC was as low as 58.9% and that of advanced GC 33.3%, agreeing with previous studies[6,8]. In particular, the prognosis of patients with early GC worsened dramatically, which is markedly different from the overall 5-year survival rate of 93.4% after surgery for T1 tumor based on the nationwide data collected by the Japanese Gastric Cancer Association[10]. The primary cause of this difference was the high mortality related to LC, especially death from HCC. Of the 25 GC patients with LC in this study, 16 had died to date. Four died of liver cancer, similar to the 5 who died of recurrent GC. In this study, in addition to overall survival analysis, GC-specific survival was analyzed in which death due to other diseases was dealt with by censoring. This analytical procedure helps understand the impact of LC-related death on patient survival more clearly. Furthermore, it is noteworthy that all the 5 patients who developed HCC in this study were those with early GC. In this study, nonsurgical therapy was selected for 3 GC patients with synchronous HCC and all of them finally died of HCC. This suggests that a more radical treatment for the HCC should have been chosen even if GC surgery was downscaled or completely omitted. Moreover, it becomes more important to discover metachronous HCC that develops during postoperative follow-up because essentially early GC patients can survive longer. In this study, 2 of 25 patients (8.0%) suffered from metachronous HCC following a gastrectomy. The incidence was very similar to the incidence of 8.5% (3/35) reported by Isozaki et al[6] The cumulative appearance rates of HCC were 18.9% and 39.5% at 5 years in HBsAg and anti-HCV-positive cirrhotic patients respectively, both of which are considerably high in Japan[14]. Although this may not be applicable to Western countries where alcoholic cirrhosis is more prevalent and the incidence of liver cancer is low, early detection of HCC is vital during patient follow-up in East Asian countries where the incidences of both GC and HCC are high.
In conclusion, radical gastrectomy can be conducted safely in GC patients with grade B cirrhosis based on the Child-Pugh classification. However, the extent of lymph node dissection should be minimized because grade B patients are at risk of postoperative intractable ascites. The prognosis of GC patients with LC is poor due to HCC. To improve patient prognosis, high-quality control and early diagnosis of HCC by intensive follow-up are vital.
It remains uncertain whether or not the Child-Pugh classification is sufficiently reliable to assess the risk of gastric cancer (GC) surgery as only a limited number of studies have been conducted. In addition, it is necessary to know the effects of liver cirrhosis (LC)-related diseases, namely hepatocellular carcinoma (HCC), on the prognosis of patients after GC surgery.
With regard to the extent of lymph node dissection in LC patients, a D2 dissection is controversial. It is essential to have more detailed information about the prognosis of GC patients with LC to establish a better therapeutic strategy.
Clinical outcomes including postoperative morbidity and survival were retrospectively analyzed based on medical records and surgical files. Radical gastrectomy can be conducted safely in GC patients with grade B cirrhosis based on the Child-Pugh classification. However, the extent of lymph node dissection should be minimized because grade B patients are at risk of postoperative intractable ascites. The prognosis of GC patients with LC is poor due to HCC. To improve patient prognosis, high-quality control and early diagnosis of HCC by intensive follow-up are vital.
The study results suggest that the risk of postoperative intractable ascites is high, particularly in grade B patients. Early detection and complete control of HCC is vital to improve a patient’s prognosis.
The Child-Pugh classification replaces assessment factors with laboratory data and scores them, and is more frequently used to evaluate overall hepatic function. Prothrombin time is added to replace the nutritional assessment as a modification of the Child’s classification.
This is a good study in which the authors report on the usefulness of risk assessment of GC surgery based on the currently used Child-Pugh classification. By investigating in detail the prognosis of patients, this report first demonstrates the effects of LC and LC-related diseases on the long-term prognosis of patients with GC.
Peer reviewer: Yun-Fei Yuan, Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, 651 Dongfeng Road E., Guangzhou 510060, Guangdong Province, China
S- Editor Li LF L- Editor Roemmele A E- Editor Lin YP
| 1. | Garrison RN, Cryer HM, Howard DA, Polk HC Jr. Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann Surg. 1984;199:648-655. |
| 2. | Fekete F, Belghiti J, Cherqui D, Langonnet F, Gayet B. Results of esophagogastrectomy for carcinoma in cirrhotic patients. A series of 23 consecutive patients. Ann Surg. 1987;206:74-78. |
| 3. | Sirinek KR, Burk RR, Brown M, Levine BA. Improving survival in patients with cirrhosis undergoing major abdominal operations. Arch Surg. 1987;122:271-273. |
| 4. | Jakab F, Ráth Z, Sugár I, Ledniczky G, Faller J. Complications following major abdominal surgery in cirrhotic patients. Hepatogastroenterology. 1993;40:176-179. |
| 5. | Takeda J, Hashimoto K, Tanaka T, Koufuji K, Kakegawa T. Review of operative indication and prognosis in gastric cancer with hepatic cirrhosis. Hepatogastroenterology. 1992;39:433-436. |
| 6. | Isozaki H H, Okajima K, Kawashima Y, Yamada S, Morita S, Nakajima T, Nakata E, Iga C, Ishibashi T, Tanimura M, Hara H. Optimal extent of lymph node dissection in the surgical treatment of gastric cancer accompanied by liver cirrhosis (in Japanese with English abstract). Nippon Shokaki Geka Gakkai Zasshi (Jpn J Gastroenterol Surg). 1991;24:798-804. |
| 7. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. |
| 8. | Jang HJ, Kim JH, Song HH, Woo KH, Kim M, Kae SH, Lee J, Cho JW, Kang JH, Lee SI. Clinical outcomes of patients with liver cirrhosis who underwent curative surgery for gastric cancer: a retrospective multi-center study. Dig Dis Sci. 2008;53:399-404. |
| 9. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 13th ed. Tokyo: Kanehara 1999; . |
| 10. | Japanese Gastric Cancer Association. Gastric cancer treatment guideline. 2nd ed. Tokyo: Kanehara 2004; . |
| 12. | Lee JH, Kim J, Cheong JH, Hyung WJ, Choi SH, Noh SH. Gastric cancer surgery in cirrhotic patients: result of gastrectomy with D2 lymph node dissection. World J Gastroenterol. 2005;11:4623-4627. |
| 13. | Ryu KW, Lee JH, Kim YW, Park JW, Bae JM. Management of ascites after radical surgery in gastric cancer patients with liver cirrhosis and minimal hepatic dysfunction. World J Surg. 2005;29:653-656. |
| 14. | Ishikawa T, Ichida T, Yamagiwa S, Sugahara S, Uehara K, Okoshi S, Asakura H. High viral loads, serum alanine aminotransferase and gender are predictive factors for the development of hepatocellular carcinoma from viral compensated liver cirrhosis. J Gastroenterol Hepatol. 2001;16:1274-1281. |