Copyright
©The Author(s) 2015.
World J Gastrointest Surg. Nov 27, 2015; 7(11): 306-312
Published online Nov 27, 2015. doi: 10.4240/wjgs.v7.i11.306
Published online Nov 27, 2015. doi: 10.4240/wjgs.v7.i11.306
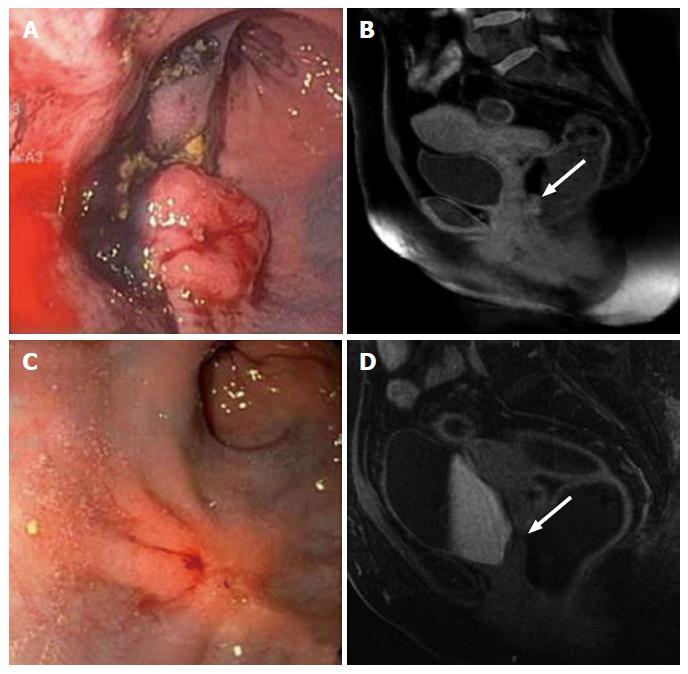
Figure 1 Clinical incomplete response.
Evaluation of the rectal cancer prior to the initiation of neoadjuvant chemoradiation therapy by flexible sigmoidoscopy (A) and MRI (B, white arrow: Tumor). Evaluation of 7 wk after completion of neoadjuvant chemoradiation therapy. The tumor has decreased in size; however, it continues to be present as evidenced by flexible sigmoidoscopy (C) and MRI (D, white arrow: Tumor). MRI: Magnetic resonance imaging.
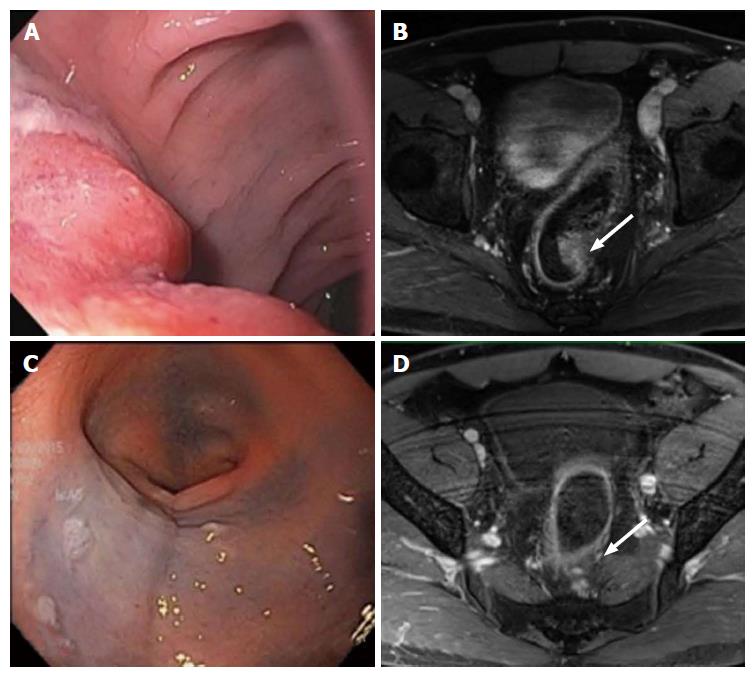
Figure 2 Complete clinical response.
Evaluation of the rectal cancer prior to the initiation of neoadjuvant chemoradiation therapy by flexible sigmoidoscopy (A) and MRI (B, white arrow: Tumor). Evaluation of 7 wk after completion of neoadjuvant chemoradiation therapy showed no evidence of tumor by flexible sigmoidoscopy (C) and MRI (D, white arrow: Tumor). MRI: Magnetic resonance imaging.
- Citation: Pozo ME, Fang SH. Watch and wait approach to rectal cancer: A review. World J Gastrointest Surg 2015; 7(11): 306-312
- URL: https://www.wjgnet.com/1948-9366/full/v7/i11/306.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v7.i11.306