Published online Aug 15, 2015. doi: 10.4251/wjgo.v7.i8.118
Peer-review started: April 5, 2015
First decision: May 13, 2015
Revised: May 23, 2015
Accepted: June 15, 2015
Article in press: June 16, 2015
Published online: August 15, 2015
Gastric carcinoma is derived from epithelial cells in the gastric mucosa. We reported an extremely rare case of submucosal gastric carcinoma originating from the heterotopic submucosal gastric gland (HSG) that was safely diagnosed by laparoscopy and endoscopy cooperative surgery (LECS). A 66-year-old man underwent gastrointestinal endoscopy, which detected a submucosal tumor (SMT) of 1.5 cm in diameter on the lesser-anterior wall of the upper gastric body. The tumor could not be diagnosed histologically, even by endoscopic ultrasound-guided fine-needle aspiration biopsy. Local resection by LECS was performed to confirm a diagnosis. Pathologically, the tumor was an intra-submucosal well differentiated adenocarcinoma invading 5000 μm into the submucosal layer. The resected tumor had negative lateral and vertical margins. Based on the Japanese treatment guidelines, additional laparoscopic proximal gastrectomy was curatively performed. LECS is a less invasive and safer approach for the diagnosis of SMT, even in submucosal gastric carcinoma originating from the HSG.
Core tip: This report describes the rare case of a submucosal gastric carcinoma originating from the heterotopic submucosal gastric gland (HSG) that was safely diagnosed by laparoscopy and endoscopy cooperative surgery (LECS). LECS is a less invasive and safer approach for the diagnosis of submucosal tumor, even in submucosal gastric carcinoma originating from the HSG.
- Citation: Imamura T, Komatsu S, Ichikawa D, Kobayashi H, Miyamae M, Hirajima S, Kawaguchi T, Kubota T, Kosuga T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H, Ogiso K, Yagi N, Yanagisawa A, Ando T, Otsuji E. Gastric carcinoma originating from the heterotopic submucosal gastric gland treated by laparoscopy and endoscopy cooperative surgery. World J Gastrointest Oncol 2015; 7(8): 118-122
- URL: https://www.wjgnet.com/1948-5204/full/v7/i8/118.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v7.i8.118
Gastric carcinoma is commonly derived from epithelial cells in the gastric mucosa and is very rarely initially diagnosed as a submucosal tumor (SMT). We herein presented a case of submucosal gastric carcinoma originating from the heterotopic submucosal gastric gland (HSG) that was safely diagnosed by laparoscopy and endoscopy cooperative surgery (LECS) and treated by subsequent laparoscopic gastrectomy with D1+ lymphadenectomy. We reviewed the clinical features of this rare tumor and selected successful decision-making using the LECS technique.
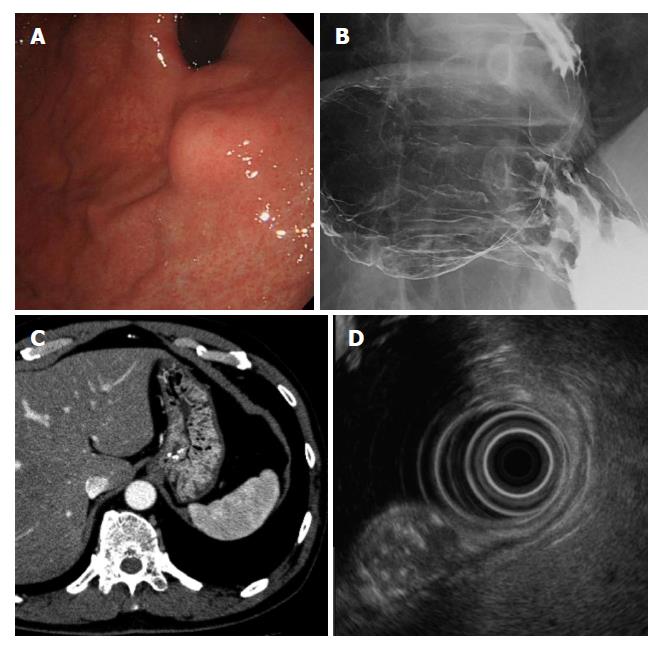
The patient was a 66-year-old man who underwent upper endoscopy in a medical checkup, which showed a SMT on the upper gastric body. The patient was referred to the hospital for diagnosis and treatment. Endoscopic re-examination detected a SMT of 15 mm in diameter on the anterior wall of the upper gastric body. The tumor did not have a depression or ulceration (Figure 1A). The results of endoscopic biopsy from the gastric mucosa on the tumor were chronic gastritis with no evidence of malignancy. Barium gastrography showed a smooth elevated lesion of 2 cm in diameter on the anterior wall of the upper gastric body near the esophago-gastric junction (Figure 1B). Computed tomography revealed a 15-mm low density area with calcification in the anterior wall of the upper gastric body and no lymph node or distant metastasis (Figure 1C). Endoscopic ultrasound (EUS) showed an 11.2 mm × 13.5 mm SMT that was derived from the third layer of the gastric wall as a heterogeneous lesion with a mixture of a high echoic lesion, low echoic lesion, and calcification (Figure 1D). The tumor could not be diagnosed histologically, even by EUS-guided fine-needle aspiration biopsy at multiple sites. LECS for gastric local resection was selected as decision-making for a pathological diagnosis and safe removal.
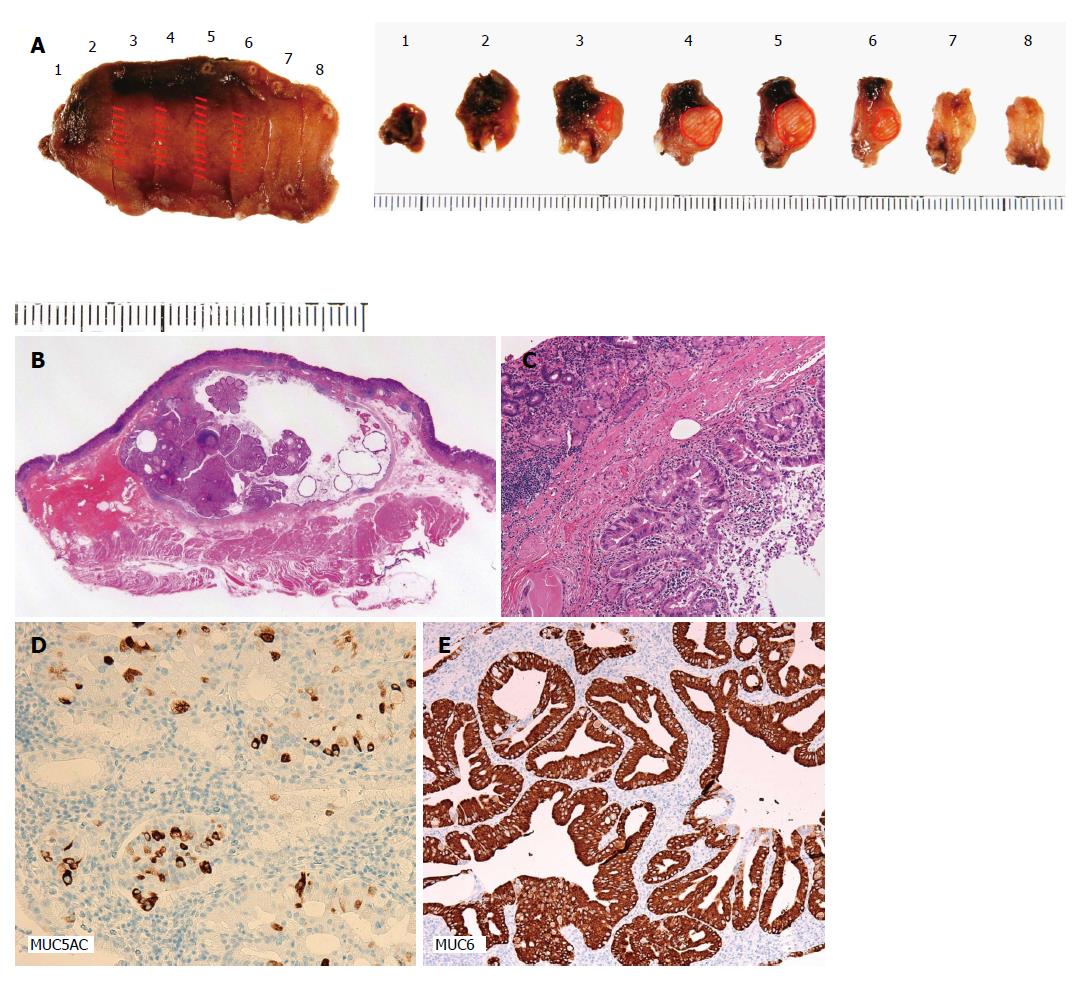
Observations in the abdominal cavity by laparoscopy confirmed no distant or nodal metastasis. The SMT was endoscopically detected on the anterior wall of the lesser curvature of the upper gastric body, but not by laparoscopy. To avoid bleeding, the peripheral branches of the left gastric artery near the tumor were coagulated using a laparoscopic ultrasonically activated device. Endoscopic submucosal resection around the tumor was performed using the endoscopic submucosal dissection technique and seromuscular dissection was performed around the tumor along the line of submucosal resection. The incisional line in the stomach was closed using a laparoscopic stapling device. The resected tumor had negative lateral and vertical margins with normal mucosa (Figure 2A). A pathological examination confirmed that the tumor was a SMT that invaded 5000 μm into the submucosal layer, measured 20 mm × 11 mm × 6 mm, and was a well differentiated adenocarcinoma (Figure 2B). Dilated gastric glands were detected in the submucosal layer (Figure 2C). There was no lymphovascular invasion. Immunohistochemical staining revealed the positive expression of MUC5AC and MUC6, indicating differentiation into the pyloric glands (Figure 2D).
Eighty-four days after LECS, additional laparoscopic proximal gastrectomy with D1+ lymphadenectomy was performed based on the Japanese Gastric Cancer Treatment Guidelines[1]. A pathological examination confirmed no residual tumor cells or lymph node metastasis. The postoperative course was uneventful and the patient is alive without recurrence 1 year after surgery.
HSG shows that cystic dilated gastric glands exist in the gastric submucosal layer and has been recognized as a benign condition occurring as a result of repeated mucosal damage[2,3]. HSG was previously described as a para-cancerous lesion found in 4% of resected specimen from the stomachs of patients with gastric carcinoma, and multiple cancers have been detected in 30% of specimens of gastric carcinoma associated with HSG[4]. However, little is known about the carcinogenesis of HSG itself. Kim et al[5] described two cases of early gastric carcinoma arising from HSG that were treated by laparoscopic gastric wedge resection. To the best of our knowledge, there have been no other studies in English concerning gastric carcinoma originating from HSG.
Table 1 shows a summary of 17 previously reported cases, including cases in Japan and our case. Gastric carcinoma originating from HSG occurred more frequently in males and in the middle area of the stomach. Regarding histological findings, the well differentiated type was more common. A study has not yet been conducted on lymph node metastasis from gastric carcinoma originating from HSG. This summary showed that more than 65% of patients could not be histologically diagnosed by biopsy and FNA using EUS before resection.
| Total number of reported cases | n | (%) | |
| 17 | |||
| Age | 64.1 (45-81) | ||
| Sex | Male | 11 | 65 |
| Female | 6 | 35 | |
| Location | Upper | 4 | 24 |
| Middle | 8 | 47 | |
| Lower | 5 | 29 | |
| Size (mm) | 20.5 (8-50) | ||
| Ulceration or depression | Present | 13 | 76 |
| Absent | 4 | 24 | |
| Histological type | Well differentiated | 16 | 94 |
| Unknown | 1 | 6 | |
| Depth of invasion | m | 1 | 6 |
| sm | 14 | 82 | |
| T2 or more | 2 | 12 | |
| Diagnosis by biopsy | Present | 6 | 35 |
| Absent | 11 | 35 | |
| EUS-FNA | Present | 2 | 12 |
| Absent | 15 | 88 | |
| Treatment | EMR | 1 | 6 |
| EMR and surgical resection | 3 | 18 | |
| Surgical resection | 12 | 71 | |
| LECS + surgical resection | 1 | 6 |
The recent development of endoscopic and laparoscopic techniques has allowed for less invasive diagnoses and treatments. LECS is a novel and excellent approach for local gastric resection, and was developed by Hiki et al[19] as an alternative strategy to laparoscopic wedge resection for gastric SMT. The feasibility and safety of this procedure for gastric SMT have been demonstrated in several studies[20-22]. LECS is now being applied to the treatment of early gastric cancer[23]. The most critical issue associated with its application to gastric cancer is the dissemination of cancer cells into the peritoneal cavity during surgery. Therefore, several methods have been investigated for LECS[24-26]. LECS is a promising approach for the diagnosis of SMT, even in gastric carcinoma originating from HSG.
A 66-year-old man who underwent upper endoscopy in a medical checkup, which showed a submucosal tumor (SMT) on the upper gastric body.
The presented patients had submucosal gastric tumor that could not be diagnosed histologically by endoscopic biopsy.
Gastrointestinal stromal tumor, early gastric tumor, smooth muscle tumor.
There were no abnormal findings in laboratory examinations including tumor markers.
Endoscopic ultrasound and computed tomography showed that the tumor was derived from the third layer of the gastric wall.
Pathological examination confirmed that the tumor was an intra submucosal tumor that was a well differentiated adenocarcinoma.
Laparoscopy and endoscopy cooperative surgery (LECS) for gastric local resection was selected as decision-making for a pathological diagnosis and safe removal.
LECS: Laparoscopy and endoscopy cooperative surgery; HSG: Heterotopic submucosal gastric gland.
Gastric carcinoma originating from the HSG forms a submucosal gastric tumor and is often difficult to diagnose by endoscopic biopsy. If unable to deny malignant disease, resection of the tumor should be considered.
This manuscript described a rare case of submucosal gastric carcinoma originating from the HSG and the authors also described the treatment of the carcinoma by LECS.
P- Reviewer: Marin JJG, Yang SM S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1723] [Cited by in F6Publishing: 1844] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 2. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2390] [Cited by in F6Publishing: 2656] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 3. | Morozumi A, Ikeda A, Fujino M. A case of well differentiated adenocarcinoma of the stomach forming a peculiar-shaped mass probably originating from ectopic gastric gland. ENDOSCOPIC FORUM for digestive disease. 1988;4:310-315. [Cited in This Article: ] |
| 4. | Iwanaga T, Koyama H, Takahashi Y, Taniguchi H, Wada A. Diffuse submucosal cysts and carcinoma of the stomach. Cancer. 1975;36:606-614. [PubMed] [Cited in This Article: ] |
| 5. | Kim DH, Kim KM, Oh SJ, Oh JA, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S. Early gastric cancer arising from heterotopic gastric mucosa in the gastric submucosa. J Korean Surg Soc. 2011;80 Suppl 1:S6-S11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Yamaoku K, Kunisaki C, Sato T. A Case of Gastric Cancer Resembling a Submucosal Tumor Difficulty to Diagnose Preoperatively with a Review of 183 Case. J Jpn Coll Surg. 2011;36:623-632. [Cited in This Article: ] |
| 7. | Yaosaka T, Tsukagoshi H, Suga T. Analysis of gastric cancer being a form of submucosal tumor. Gastroenterological Endoscopy. 1977;19:814. [Cited in This Article: ] |
| 8. | Hayabe Y, Hajiro K, Tsujimura D. Early gastric cancer resembling submucosal tumor: a case report and review of the literature. Gastroenterological Endoscopy. 1989;31:2440-2445. [Cited in This Article: ] |
| 9. | Mitsuno M, Tsukiyama M, Yoshioka K. A case of early gastric cancer originating from heterotopic gastric gland. J Oky Surg Path. 1989;26:69-74. [Cited in This Article: ] |
| 10. | Koyama H, Koyama N, Itoh H. A case of small gastric cancer diagnosed as a submucosal tumor. Stomach and Intestine. 1995;30:819-822. [Cited in This Article: ] |
| 11. | Ohta K, Maruyama T, Anezaki K. A case of early gastric cancer originating from heterotopic gastric gland in gastric submucosa. ENDOSCOPIC FORUM for digestive disease. 2000;16:25-28. [Cited in This Article: ] |
| 12. | Ohira S, Hasegawa H, Kogiso S. A case of early gastric cancer derived from heterotopic submucosal gastric gland. Stomach and Intestine. 2002;37:233-237. [Cited in This Article: ] |
| 13. | Abo T, Satoh T, Imamura T. 5-year-follow up of gastric cancer originating from heterotopic gastric gland in gastric submucosa. Stomach and Intestine. 2003;38:1551-1556. [Cited in This Article: ] |
| 14. | Yoshimitsu S, Tada S, Goda K. Three cases of diffuse cystic malformation accompanied by early gastric cancer. Gastroenterological Endoscopy. 2003;45:1036-1043. [Cited in This Article: ] |
| 15. | Nishinaka H, Kodama K, Iwamura M. A case of gastric cancer derived from heterotopic gastric gland in gastric wall. Stomach and Intestine. 2003;38:1562-1566. [Cited in This Article: ] |
| 16. | Fukuda N, Oda S, Tsujigami K. A case of an early gastric cancer resembling submucosal tumor derived from heterotopic gastric gland. Gastroenterological Endoscopy. 2007;499:2498-2503. [Cited in This Article: ] |
| 17. | Kanamori H, Sasaki M, Ogasawara N. A case of advanced gastric cancer of submucosal form, originating from submucosal heterotopic gastric glands simultaneously with hepatocellular carcinoma. Gastroenterological Endoscopy. 2011;53:255-261. [Cited in This Article: ] |
| 18. | Nakamatsu D, Nishida T, Inoue T. Advanced gastric cancer deriving from submucosal heterotopic gastric glands based on pathological diagnosis. J jpn Gastroenterol. 2013;110:290-293. [Cited in This Article: ] |
| 19. | Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729-1735. [PubMed] [Cited in This Article: ] |
| 20. | Tsujimoto H, Yaguchi Y, Kumano I, Takahata R, Ono S, Hase K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg. 2012;36:327-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Kawahira H, Hayashi H, Natsume T, Akai T, Uesato M, Horibe D, Mori M, Hanari N, Aoyama H, Nabeya Y. Surgical advantages of gastric SMTs by laparoscopy and endoscopy cooperative surgery. Hepatogastroenterology. 2012;59:415-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Hoteya S, Haruta S, Shinohara H, Yamada A, Furuhata T, Yamashita S, Kikuchi D, Mitani T, Ogawa O, Matsui A. Feasibility and safety of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors, including esophagogastric junction tumors. Dig Endosc. 2014;26:538-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Hiki N, Nunobe S, Matsuda T, Hirasawa T, Yamamoto Y, Yamaguchi T. Laparoscopic endoscopic cooperative surgery. Dig Endosc. 2015;27:197-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 24. | Nunobe S, Hiki N, Gotoda T, Murao T, Haruma K, Matsumoto H, Hirai T, Tanimura S, Sano T, Yamaguchi T. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer. 2012;15:338-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Inoue H, Ikeda H, Hosoya T, Yoshida A, Onimaru M, Suzuki M, Kudo SE. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am. 2012;21:129-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Mitsui T, Goto O, Shimizu N, Hatao F, Wada I, Niimi K, Asada-Hirayama I, Fujishiro M, Koike K, Seto Y. Novel technique for full-thickness resection of gastric malignancy: feasibility of nonexposed endoscopic wall-inversion surgery (news) in porcine models. Surg Laparosc Endosc Percutan Tech. 2013;23:e217-e221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |