Published online Apr 15, 2010. doi: 10.4251/wjgo.v2.i4.197
Revised: February 22, 2010
Accepted: March 1, 2010
Published online: April 15, 2010
AIM: To assess the relationship between preoperative computed tomography (CT) and postoperative pathological measurements of esophageal tumor length and the prognostic significance of CT tumor length data.
METHODS: A retrospective study was carried out in 56 patients who underwent curative esophagogastrectomy. Tumor lengths were measured on the immediate preoperative CT and on the post-operative resection specimens. Inter- and intra-observer variations in CT measurements were assessed. Survival data were collected.
RESULTS: There was a weak correlation between CT and pathological tumor length (r = 0.30, P = 0.025). CT lengths were longer than pathological lengths in 68% (38/56) of patients with a mean difference of 1.67 cm (95% CI: 1.18-2.97). The mean difference in measurements by two radiologists was 0.39 cm (95% CI: -0.59-1.44). The mean difference between repeat CT measured tumor length (intra-observer variation) were 0.04 cm (95% CI: -0.59-0.66) and 0.47 cm (95% CI: -0.53-1.47). When stratified, patients not receiving neoadjuvant chemotherapy showed a strong correlation between CT and pathological tumor length (r = 0.69, P = 0.0014, n = 37) than patients that did (r = 0.13, P = 0.43, n = 19). Median survival with CT tumor length > 5.6 cm was poorer than with smaller tumors, but the difference was not statistically significant.
CONCLUSION: Esophageal tumor length assessed using CT does not reflect pathological tumor extent and should not be the only modality used for management decisions, particularly for planning radiotherapy.
- Citation: Sillah K, Williams LR, Laasch HU, Saleem A, Watkins G, Pritchard SA, Price PM, West CM, Welch IM. Computed tomography overestimation of esophageal tumor length: Implications for radiotherapy planning. World J Gastrointest Oncol 2010; 2(4): 197-204
- URL: https://www.wjgnet.com/1948-5204/full/v2/i4/197.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v2.i4.197
Tumor length is a recognised independent adverse prognostic factor following surgery or radiotherapy in patients with esophageal cancer[1-3]. In a study by Griffiths et al[2], patients with pathological tumor length > 3.5 cm had a poorer prognosis than those with shorter tumors following esophagogastrectomy for cancer. Tumor dimensions, including length, are included in the United Kingdom Royal College of Pathologist’s minimum dataset for the histopathological reporting of the disease[4]. As such, its accurate measurement preoperatively would provide essential information for treatment planning and prognosis.
Contrast enhanced computed tomography (CT) is an important aspect of staging patients with esophageal cancer[5-8]. However, published studies on the relationship between tumor lengths reported on the preoperative CT scan and the corresponding length on the postoperative pathological specimen are inconsistent. Using pathological length as the gold standard and discounting length differences of 1 cm or less, Drudi et al[9] reported 32% concordance between the two measurement types and CT was found to under-estimate tumor length. However, Quint et al[10] reported CT generally overestimated tumor length by 1.5-7.5 cm. This finding was echoed by Gao et al[11] in which 34 patients with middle and distal third esophageal squamous cell carcinoma were found to have longer tumor length on the preoperative CT scans with a mean CT length of 4.48 cm vs mean pathological length of 3.82 cm.
The extent of gross esophageal tumor impacts on the surgeon’s choice of operative approach. Also, delineation of gross tumor volume (GTV) for radiotherapy is reliant on a CT scan performed for the purposes of radiotherapy planning, supplemented by information obtained from clinical staging procedures. The clinical target volume (CTV) will encompass the GTV and additional tissue based on pathological extent of subclinical disease from resected surgical series[11]. It is, therefore, essential to ascertain as accurately as possible the gross tumor extent and staging by CT, barium imaging, endoscopy and endoscopic ultrasound (EUS).
The aims of this work were to compare the level of agreement between pathological and CT length of resected esophageal adenocarcinoma and evaluate the implication of any inter- and intra-observer variation in CT measurement. We also assessed any association between tumor length and other clinico-pathological factors and the degree of contrast distension of the stomach or esophagus. In principle, good visceral distension by contrast agent should help delineate tumor dimensions but whether this plays a role in optimising the accuracy of CT measurement of tumor length is not clear. The study also investigated the prognostic value of tumor length measured preoperatively using CT.
The study was retrospective and involved 56 patients who underwent esophagogastrectomy for cancer between September 1999 and June 2007 at the University Hospital of South Manchester, UK. There were eight females (14%) and 48 males (86%), and the median age was 65 years (range 36-82 years). Sixty-six percent (37/56) of the patients underwent neoadjuvant chemotherapy. In these cases, the post chemotherapy/pre-surgery scan was used. All tumors were adenocarcinomas of the distal third of the esophagus. Ethical approval for the study was obtained from the South Manchester Research Ethics Committee.
All 56 patients underwent intravenous and oral contrast enhanced preoperative staging CT scans of the thorax and upper abdomen. The subjects were scanned at several referring hospitals using a variety of scanners. Different oral contrast preparations were utilised: Gastrografin™ based solution in 55 patients and water based, negative oral contrast in one patient. Slice thickness ranged from 4-10 mm. The CT carried out closest to the time of tumor resection was reviewed in each case. The mean time from the date of scanning to surgery was 37 d (range 1-149 d). All images were reviewed on hard copy axial images.
The slices judged to be tumor free superior and inferior to the cancer were identified, allowing a judgement of the cranio-caudal extent of the tumor. The maximal esophageal wall thickness, maximal esophageal diameter, esophageal distension, gastric distension and presence of hiatus hernia were all recorded.
The cranio-caudal tumor lengths in the immediate preoperative contrast enhanced axial CT images were estimated independently by two radiologists. A senior trainee radiologist assessed the scans of 56 patients and a specialist consultant radiologist assessed 42 of the 56 patients. The CT tumor lengths of a cohort of the patients were subsequently re-estimated independently by both radiologists. This allowed evaluation of inter- and intra-observer variation.
During the study period, all esophagogastrectomy specimens from the operating theatres were immersed in formalin and sent to the Department of Histopathology for analysis. On receipt of the specimen, the pathologists inked the circumferential resection margin (CRM). The esophagus was subsequently opened along its longitudinal axis from proximal to distal extending along the greater curvature of the stomach. The opened specimen was then fixed in formalin for at least 24 h.
Information on whether the specimens were pinned or unpinned was not available. The histopathological tumor details of all the patients in the study were obtained from the computed pathology records. Macroscopic parameters were recorded as detailed in the United Kingdom Royal College of Pathologists minimum dataset for the histopathological reporting of esophageal cancer[4].
Statistical analysis was performed using SPSS® (SPSS, Chicago, Illinois, USA) Version 11.5 and Stata® (StataCorp, 4905 Lakeway Drive, College Station, TX 77845 USA) Version 9.2. One-way analysis of variance (ANOVA), the Mann-Whitney or Spearman’s tests were used to assess factors associated with tumor length. Agreement between pathological and CT lengths for each radiologist and inter-and intra-observer variation was assessed using the Bland-Altman plot[12]. Survival was defined as the time from the date of surgery until death or most recent follow up appointment and was analysed using log rank test. A P≤ 0.05 was considered to be statistically significant.
Tables 1 and 2 summarise the clinico-pathological and radiological details of the 56 patients. No significant associations were found between CT or pathology measurements of tumor length and patients’ age, gender, tumor morphology, differentiation, overall histological stage, resection category (complete microscopic clearance vs microscopic evidence of tumor cells at the resection margins), presence of hiatus hernia, slice thickness and the degree of the gastro-esophageal contrast distension. However there was a significant correlation between maximal esophageal thickness and tumor length on the CT scan (P = 0.01).
| Variable | n | P |
| Mean tumor length (range) (cm) | ||
| 4.2 (0-11.5) | ||
| Median age (range) years (yr) | ||
| 65 (36-82) | 56 | 0.121 |
| Gender | ||
| Male | 48 | 0.922 |
| Female | 8 | |
| Neoadjuvant chemotherapy3 | ||
| No | 19 | 0.644 |
| Yes | 37 | |
| Tumor type | ||
| Adenocarcinoma | 56 | |
| Tumor morphology | ||
| Polypoidal | 14 | 0.074 |
| Stenosing | 15 | |
| Ulcerating | 27 | |
| Differentiation | ||
| Well | 4 | 0.361 |
| Moderate | 32 | |
| Poor | 20 | |
| T-stage | ||
| T1 | 8 | 0.181 |
| T2 | 13 | |
| T3 | 34 | |
| T4 | 1 | |
| N-stage | ||
| N0 | 19 | 0.122 |
| N1 | 37 | |
| M-stage | ||
| M0 | 54 | |
| M1a | 2 | |
| Resection category | ||
| R0 | 47 | 0.222 |
| R1 | 9 |
| Variable | n | P |
| Mean tumor length (range)/(cm) | ||
| 5.9 (0-15) | 56 | |
| Mean maximum esophageal thickness (range)/(cm) | ||
| 3.1 (1.7-4.7) | 56 | 0.011 |
| Mean slice thickness (range)/(mm) | ||
| 5.5 (4-10) | 56 | 0.25 |
| Presence of hiatus hernia | ||
| Yes | 9 | 0.762 |
| No | 47 | |
| Degree of esophageal distension | ||
| Good | 16 | 0.831 |
| Moderate | 16 | |
| Poor | 24 | |
| Degree of gastric distension | ||
| Good | 23 | 0.741 |
| Moderate | 22 | |
| Poor | 11 |
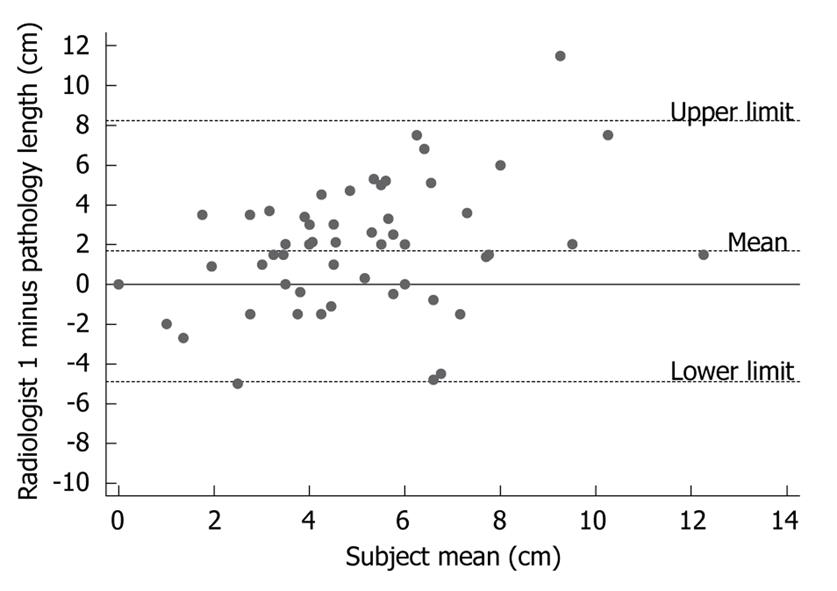
There was a weak positive correlation between CT and pathology measurements of esophageal tumor lengths (r = 0.30, P = 0.02, n = 56). However, the Bland-Altman plots in Figure 1 illustrate the generally poor level of agreement between CT measurements of esophageal tumor lengths and their corresponding pathological lengths. In the Bland-Altman plot, the difference between the two measurements is plotted against their mean-the best estimate of the true value. If there is good agreement between the methods, then the results would be close to zero. Pre-operative CT length was longer than post-operative pathology length in 68% (38/56) of the patients with an average tumor length difference of 1.67 cm (95% CI: 1.18-2.97). The difference in tumor length between CT and pathological measurements was statistically significant (P < 0.0005, n = 56, paired t-test). There was no relationship between the time from CT scan to surgery (median 37 d, range 1-149 d) and the difference in CT and pathological tumor length (r = -0.16, P = 0.25).
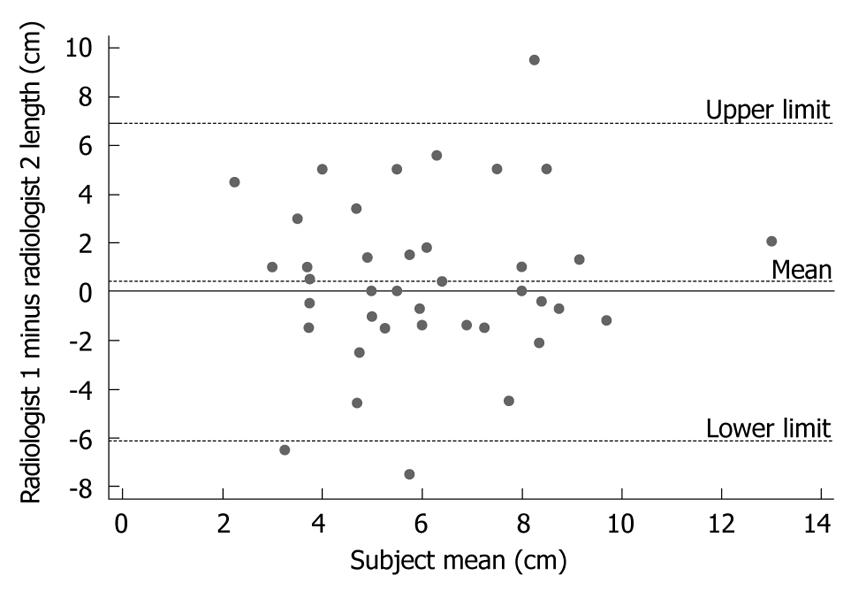
In order to address whether the poor agreement between CT and pathology measurements of tumor length was due to a poor reliability in obtaining CT data, a second radiologist measured tumor length in 42 of the patients. Qualitatively similar results were found to the first radiologist. There was a weak positive correlation between CT and pathological tumor length (r = 0.20, P = 0.92, n = 42). The average tumor length difference (CT minus pathology) was 1.52 cm (95% CI: 0.62-2.68), and CT length was longer in 69% (29/42) of the patients (P = 0.006, n = 42, paired t-test). Agreement between the two radiologists was summarised by calculating the mean difference between their measurements (Figure 2). This analysis shows a small inter-observer difference in measurements of tumor length with a mean difference in the measured tumor length between the two radiologists’ being 0.39 cm (95% CI: -0.59-1.44). However, a large spread in the observations was observed, with radiologist 2 measuring one tumor 6 cm shorter and another tumor 7 cm longer than radiologist 1. As this lack of agreement between CT and pathology measurements can be attributed, at least in part, to variability in obtaining CT measurements, an assessment was made of intra-individual variability in measuring tumor length.
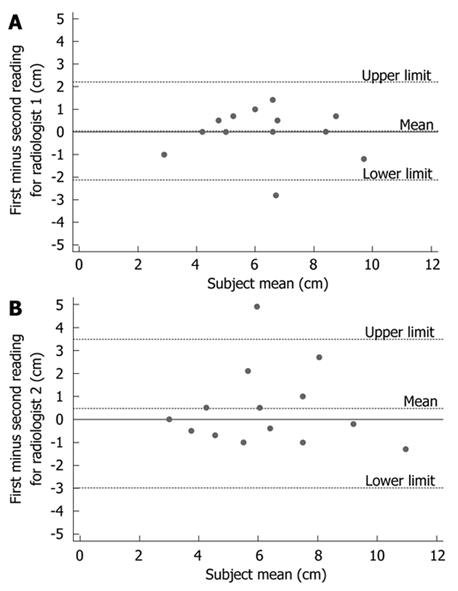
Table 3 summarises the two separate tumor length readings by the two radiologists with the corresponding pathological lengths and Figure 3 illustrates the Bland-Altman plot for intra-observer variation or repeatability. For radiologist 1 the mean difference between the two scores was 0.04 cm (95% CI: -0.59-0.66). For radiologist 2, the mean difference was 0.47 cm (95% CI: -0.53-1.47). The large 95% CIs for both radiologists suggests it is difficult to measure reliably preoperative esophageal tumor length using CT.
| Patient | Pathology | 1st reading of Radiologist 1 | 2nd reading of Radiologist 1 | 1st reading of Radiologist 2 | 2nd reading of Radiologist 2 |
| 1 | 3.0 | 5.0 | 5.0 | 5.0 | 6.0 |
| 2 | 5.0 | 5.3 | 8.1 | 6.7 | 4.6 |
| 3 | 5.0 | 7.0 | 6.5 | 7.0 | 8.0 |
| 4 | 1.5 | 2.4 | 3.4 | 3.5 | 4.0 |
| 5 | 4.0 | 9.1 | 8.4 | 8.4 | 3.5 |
| 6 | 4.0 | 5.0 | 4.5 | 4.5 | 4.0 |
| 7 | 2.7 | 4.2 | 4.2 | 3.0 | 3.0 |
| 8 | 4.2 | 5.6 | 4.9 | 4.2 | 4.9 |
| 9 | 7.0 | 8.4 | 8.4 | 9.1 | 9.3 |
| 10 | 0 | 6.5 | 5.5 | 8.0 | 7.0 |
| 11 | 4.0 | 6.6 | 6.6 | 6.2 | 6.6 |
| 12 | 3.5 | 5.6 | 4.9 | 6.3 | 5.8 |
| 13 | 5.5 | 9.1 | 10.3 | 10.3 | 11.6 |
| 14 | 4.0 | 7.3 | 5.9 | 9.4 | 6.7 |
When patients were stratified into whether they underwent neoadjuvant chemotherapy (n = 19) or not (n = 37), a strong correlation was found between preoperative CT and postoperative pathological measurement of tumor length in the latter (r = 0.69, P = 0.001, n = 37) but not the former (r = 0.13, P = 0.43, n = 19). Bland-Altman analysis for inter-observer variability revealed a smaller mean tumor length difference between the two radiologists and relatively tighter 95% confidence intervals in the group with no neoadjuvant chemotherapy (0.27 cm; 95% CI: -1.05-1.59) than those who underwent preoperative chemotherapy (0.36 cm ; 95% CI: -136-2.08).
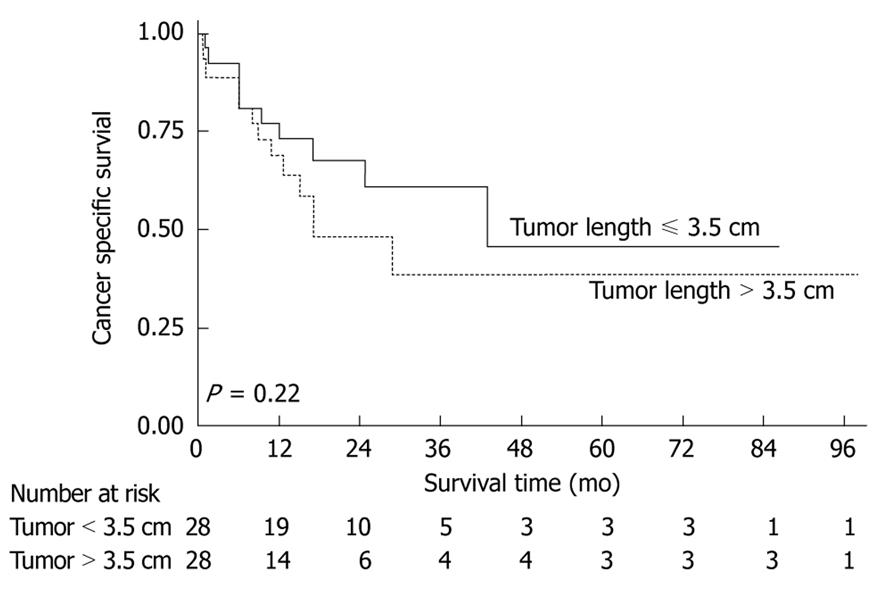
The median CT tumor length was 5.6 cm (n = 56). Despite the difficulty in obtaining repeatable CT measurements of tumor length, survival analysis revealed that the patients with tumor length ≤ 5.6 cm (n = 28) and > 5.6 cm (n = 28) had median survival times of 44 mo and 17 mo respectively. Although the survival difference was large it was not statistically significant (Figure 4).
In this study, we found no association between preoperative contrast enhanced CT defined tumor length and clinico-pathological factors. The preoperative cranio-caudal axial CT images of esophageal tumor lengths were longer than their corresponding pathological lengths following esophagogastrectomy in approximately two-thirds of the patients. In addition, results of CT measurement of tumor lengths estimated independently by the two radiologists showed marked inter- and intra-observer variability.
The proximal and distal resection margins of various tumor types have been reported to shrink significantly following formalin fixation. Some example are breast[13], colorectal[14], esophageal cancers[15] and cervical intra-epithelial neoplasia[16]. However, in all such cases, the proportion of size shrinkage attributable to the tumor itself is negligible[13-16]; and in the case of esophageal cancer, Siu et al[15] reported only 8% shrinkage in the formalin fixed specimen compared to tumor length in-vivo. This suggests that longitudinal tumor length measured in formalin fixed resected specimens is comparable to their non-fixed in-situ state.
The tendency for CT to overestimate esophageal tumor length compared to the corresponding pathological length was also reported by Gao et al[11]. They studied 34 patients with middle and distal third esophageal squamous cell carcinoma and found a statistically significant difference between the two measurement types (mean CT length of 4.48 cm vs mean pathological length of 3.82 cm, P < 0.05). This is in keeping with our finding of mean CT and pathological tumor lengths of 5.9 and 4.2 cm respectively. However, in a study of 22 patients, Drudi et al[9] found tumor length on the preoperative CT to be consistently shorter than the corresponding lengths on the resected specimens. The reasons for this finding were not discussed but may be related to observer error, scanning methodology or inadequate tumor delineation by contrast agent.
Although contrast CT was found to generally over-estimate esophageal tumor length, the difference with pathological tumor length measurement was less marked in the cohort of patients who did not undergo neoadjuvant chemotherapy. In contrast, the correlation coefficient and inter-observer agreement were both weaker in the group of patients who had neoadjuvant chemotherapy. This finding illustrates the inherent unreliability of contrast CT in assessing tumor length particularly following preoperative chemotherapy.
A number of studies have highlighted the deficiencies of CT in assessing the extent of post chemotherapy esophageal tumor bulk regression. In these studies, the radiological response rates were significantly lower than the pathological response rate to chemotherapy resulting in an apparent upward tumor stage migration[17-20]. This in part is due to chemotherapy associated inflammatory and fibrotic changes[17].
Several factors might contribute to the differences in tumor lengths obtained using the two approaches. Such factors include the difficulty in distinguishing tumor from mural thickening resulting from peri-tumor fibrosis, edema and gastric folds at the gastro-esophageal junction by CT[17,21,22]. Other possible factors contributing to disparity between CT and pathological tumor lengths are sub-optimal coating of the mucosa by contrast agent[23], movement artefacts during the scanning process such as the respiratory or cardiac cycle[24,25] and difficulty in determining the macroscopic proximal and distal limits of the tumor radiologically. Some of these factors might also contribute to the intra-observer variability in measuring tumor length using CT.
An important implication of this study is the selection of patients for appropriate treatment. Surgical data have shown that longer tumors are associated with increasing T-stage, nodal metastasis, worse overall TNM stage and increased tumor involvement of the resection margins[2]. In addition to selection for treatment and staging, CT is also routinely used as part of the radiotherapy planning process but protocols usually do not include the use of oral contrast medium. As larger radiation treatment volumes are associated with higher radiation doses to normal tissues such as the lungs[26-30] resulting in a higher incidence of treatment related morbidity and a poor therapeutic index, radiation therapy protocols usually exclude patients with longer tumor lengths (> 7-10 cm) from receiving radical radiotherapy. Since overestimation of in vivo tumor length has been commonly observed in our study, we would urge caution in excluding patients from radical radiotherapy treatment on the basis of CT findings alone. It is recommended that determination of treatment intent and target delineation of esophageal tumors during radiotherapy planning should be based on at least a further modality in addition to CT scanning as in the SCOPE (Study of Chemo-radiotherapy in Oesophageal cancer Plus or Minus Erbitux) clinical trial, currently recruiting in the UK. In the SCOPE study, where patients with a total tumor length greater than 10 cm are excluded from radical treatment, an endoscopic ultrasound (EUS) is required in addition to CT for determination of tumor length[31]. This stems from the observation that EUS more accurately measures esophageal tumor length and this imaging modality has been investigated in a number of studies[32,33].
Target definition during radiotherapy planning must be not only precise but also reliable and reproducible to avoid a geographical tumor miss and minimise the volume of normal tissue irradiated. Accurate target definition would also allow radiation dose-escalation studies to be carried out which may potentially improve clinical outcome. In a trans-Canada study in which 58 radiation oncologists were independently asked to determine the cranio-caudal length and CTV of esophageal tumors using CT images, Tai et al[34] found significant inter-observer variations in the target volume dimensions and also reported low longitudinal overlap of the CTV among the oncologists. They went on to show that specific training of oncologists could reduce the variations seen[35]. Our study echoes the implications of this finding in that imprecise target definition, as evidenced here by marked inter- and intra-observer variability of tumor length, may reduce the potential benefits of three-dimensional radiotherapy planning and high precision radiation dose delivery. A multidisciplinary approach in target delineation with close co-operation between the radiation oncologist and specialist gastro-intestinal CT radiologists may be helpful in this context.
Importantly, the use of positron emission tomography in combination with CT (PET-CT) in this setting could potentially ameliorate some of the limitations of CT alone, such as distinguishing a metabolically active tumor from peri-tumor edema, fibrosis and normal gastric rugal folds adjacent to subcardial tumors[36-38]. However, as PET acquisition is over several minutes, the tumor extent seen on PET images will include the tumor motion due to movement and patient breathing during the period, in contrast to helical or multi-detector CT scans, which are acquired in a few seconds. Methods such as gating of PET images may be helpful in this context.
Finally, survival analysis revealed that patients with CT tumor length ≤ 5.6 cm had a median survival of 44 mo compared with 17 mo for those with tumor lengths > 5.6 cm (P = 0.22). This finding, albeit based on a small number of preoperative CT length measurements, concurs with published work indicating that patients with tumor length > 3.5 cm recorded from the postoperative pathological specimen have a worse prognosis[2]. The failure of our survival analysis to achieve statistical significance may be related to the small number of patients studied. However, the marked survival time difference between the two groups of patients (17 mo vs 44 mo) suggests that accurate measurement of tumor length on the preoperative CT scan in a bigger series could provide useful prognostic information. A sample size of approximately 100 in each arm would be required to have 80% power to detect a difference in survival using the log-rank test with a 50% and 70% survival after 12 mo (assuming a constant hazard ratio of 1.9 and a conventional significance level of 0.05).
The strength of this study lies in its relatively large number of patients compared with other series, the involvement of specialist radiologists to measure tumor length and establishing, in an objective way, intra- and inter-observer variability in the radiological reporting of esophageal tumor length. The two key limitations lie in its retrospective nature and the lack of softcopy review of scans performed on a variety of scanners in referring district general hospitals. Only axial images were reviewed and many were performed on single slice scanners (11 of 56 patients). Where scans were performed on multi-slice scanners, only hard copy axial images were available for review from the referring hospitals.
This study suggests that staging CT assessments generally overestimate macroscopic esophageal tumor length particularly when tumor length is assessed following neoadjuvant chemotherapy. CT measurements of tumor length can not, therefore, act as a surrogate for pathological measurement. The limitation of CT should be considered when it is used for staging, in the selection of treatment and in radiotherapy planning. In particular, inter- and intra-observer variability in CT reporting of esophageal tumor length may result in inappropriate radiotherapy target volume delineation if based on CT scan data alone. Consequently, decisions on whether patients should have palliative or curative radiotherapy may need to be revised. The situation may be ameliorated by supporting information on tumor length provided by complementary modalities and close multidisciplinary collaboration between clinical oncologists, surgeons and radiologists with specialist interest in esophageal cancer.
Tumor length is a recognised predictor of survival in resected esophageal cancer. Studies have shown that patients with longer tumors in the resected specimens have a worse prognosis than those with shorter ones; and this is independent of advanced T-stage, lymph node metastasis and resection margin involvement with cancer cells. Consequently, accurate measurement of tumor length prior to surgery using standard imaging modalities such as computed tomography (CT) may potentially serve two purposes. First, it would provide additional prognostic information prior to surgery. Second, it would enable oncologists to more accurately calculate tumor target volumes for radiotherapy delivery. This study looked at the correlation between esophageal tumor length measured on the preoperative CT scan and the length on the postoperative pathological length (considered the gold standard).
CT is widely used to stage esophageal cancer. It is also used by radiation oncologists to determine tumor dimensions including tumor length for radiotherapy planning. The research hot spot, therefore, is to investigate the accuracy of CT in determining tumor length and factors that influence the accuracy.
Previous studies correlating tumor length measured using CT (CT tumor length) and on resected specimen (pathological tumor length) did not evaluate inter- and intra-observer variability. In this study, CT tumor lengths were measured independently by two radiologists and each radiologist measured the same tumor on two different occasions. The key findings were: (1) CT measurements of tumor length are longer than those obtained from pathology specimens, even after allowing for an effect of formalin fixation; and (2) there is a weak correlation between measurements obtained by two radiologists and repeat measurements made by the same radiologist on the same CT image, i.e. inter- and intra-observer variability hampers accurate measurements of esophageal tumor length.
The study has a number of implications. First, the work highlights the need for a review of the indications for palliative radiotherapy. This study suggests that CT tends to over-estimate the true length of esophageal tumors. In some centres, if the CT tumor length is longer than 7-10 cm, patients are considered unsuitable for radiotherapy with curative intent. Such patients might be incorrectly managed with palliative intent. Second, the high inter- and intra-observer variations in measuring tumor length using CT suggest that in some cases tumor will be excluded from the radiotherapy field-reducing the probability of local control. Third, in most cases CT over-estimation of esophageal tumor length would lead to larger volumes irradiated than required, which would increase the risk of radiation injury to important adjacent organs such as the heart and the lungs.
GTV or the gross tumor volume is the volume of tumor that can be seen on imaging (CT scan) or is visible (by endoscopy etc.) or can be felt. This is the demonstrable extent and location of the malignant growth. CTV or the clinical target volume is the gross tumor volume plus a margin allowing for microscopic spread of the disease.
This is a retrospective study in which the authors investigated the correlation between CT and pathology measurements of esophageal tumor length. All tumors were adenocarcinoma of the distal third of the esophagus. In about two-thirds of the cases, the esophageal tumor lengths measured from CT images were longer than measurements made on the resected specimen. The study also found wide variations in measurements of tumor length made by two radiologists and the same radiologist on two separate occasions. The authors conclude by highlighting the potential implications of the findings for radiotherapy planning in the treatment of esophageal cancer.
Peer reviewer: Guy Douglas Eslick, PhD, Program in Molecular and Genetic Epidemiology, Harvard School of Public Health, 677 Huntington Avenue, Building II, 2nd Floor, Boston, MA 02115, United States
S- Editor Li LF L- Editor Roemmele A E- Editor Yang C
| 1. | Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer. 2002;95:1434-1443. |
| 2. | Griffiths EA, Brummell Z, Gorthi G, Pritchard SA, Welch IM. Tumor length as a prognostic factor in esophageal malignancy: univariate and multivariate survival analyses. J Surg Oncol. 2006;93:258-267. |
| 3. | Ishikawa H, Sakurai H, Yamakawa M, Saito Y, Nakayama Y, Kitamoto Y, Okamoto M, Harada K, Hasegawa M, Nakano T. Clinical outcomes and prognostic factors for patients with early esophageal squamous cell carcinoma treated with definitive radiation therapy alone. J Clin Gastroenterol. 2005;39:495-500. |
| 4. | Mapstone NP. Dataset for the histopathological reporting of oesophageal carcinoma. 2nd ed. London: Royal College of pathologists 2007; Available from: http://www.rcpath.org/resources/pdf/G006OesophagealdatasetFINALFeb07.pdf. |
| 5. | Diederich S. Staging of oesophageal cancer. Cancer Imaging. 2007;7 Spec No A:S63-S66. |
| 6. | Greenberg J, Durkin M, Van Drunen M, Aranha GV. Computed tomography or endoscopic ultrasonography in preoperative staging of gastric and esophageal tumors. Surgery. 1994;116:696-701; discussion 701-702. |
| 7. | Hansen CP, Oskarsson K, Mortensen D. Computed tomography for staging of oesophageal cancer. Ann Chir Gynaecol. 2000;89:14-18. |
| 8. | Plukker JT, van Westreenen HL. Staging in oesophageal cancer. Best Pract Res Clin Gastroenterol. 2006;20:877-891. |
| 9. | Drudi FM, Trippa F, Cascone F, Righi A, Iascone C, Ricci P, David V, Passariello R. Esophagogram and CT vs endoscopic and surgical specimens in the diagnosis of esophageal carcinoma. Radiol Med. 2002;103:344-352. |
| 10. | Quint LE, Glazer GM, Orringer MB, Gross BH. Esophageal carcinoma: CT findings. Radiology. 1985;155:171-175. |
| 11. | Gao XS, Qiao X, Wu F, Cao L, Meng X, Dong Z, Wang X, Gao G, Wu TT, Komaki R. Pathological analysis of clinical target volume margin for radiotherapy in patients with esophageal and gastroesophageal junction carcinoma. Int J Radiat Oncol Biol Phys. 2007;67:389-396. |
| 12. | Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307-310. |
| 13. | Yeap BH, Muniandy S, Lee SK, Sabaratnam S, Singh M. Specimen shrinkage and its influence on margin assessment in breast cancer. Asian J Surg. 2007;30:183-187. |
| 14. | Goldstein NS, Soman A, Sacksner J. Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements. The effects of surgical resection and formalin fixation on organ shrinkage. Am J Clin Pathol. 1999;111:349-351. |
| 15. | Siu KF, Cheung HC, Wong J. Shrinkage of the esophagus after resection for carcinoma. Ann Surg. 1986;203:173-176. |
| 16. | Boonstra H, Oosterhuis JW, Oosterhuis AM, Fleuren GJ. Cervical tissue shrinkage by formaldehyde fixation, paraffin wax embedding, section cutting and mounting. Virchows Arch A Pathol Anat Histopathol. 1983;402:195-201. |
| 17. | Hordijk ML, Kok TC, Wilson JH, Mulder AH. Assessment of response of esophageal carcinoma to induction chemotherapy. Endoscopy. 1993;25:592-596. |
| 18. | Jones DR, Parker LA Jr, Detterbeck FC, Egan TM. Inadequacy of computed tomography in assessing patients with esophageal carcinoma after induction chemoradiotherapy. Cancer. 1999;85:1026-1032. |
| 19. | Ng CS, Husband JE, MacVicar AD, Ross P, Cunningham DC. Correlation of CT with histopathological findings in patients with gastric and gastro-oesophageal carcinomas following neoadjuvant chemotherapy. Clin Radiol. 1998;53:422-427. |
| 20. | Walker SJ, Allen SM, Steel A, Cullen MH, Matthews HR. Assessment of the response to chemotherapy in oesophageal cancer. Eur J Cardiothorac Surg. 1991;5:519-522. |
| 21. | Giovannini M, Seitz JF, Thomas P, Hannoun-Levy JM, Perrier H, Resbeut M, Delpero JR, Fuentes P. Endoscopic ultrasonography for assessment of the response to combined radiation therapy and chemotherapy in patients with esophageal cancer. Endoscopy. 1997;29:4-9. |
| 22. | Kienle P, Buhl K, Kuntz C, Düx M, Hartmann C, Axel B, Herfarth C, Lehnert T. Prospective comparison of endoscopy, endosonography and computed tomography for staging of tumours of the oesophagus and gastric cardia. Digestion. 2002;66:230-236. |
| 23. | Conces DJ Jr, Tarver RD, Lappas JC. The value of opacification of the esophagus by low density barium paste in computer tomography of the thorax. J Comput Assist Tomogr. 1988;12:202-205. |
| 24. | Lorchel F, Dumas JL, Noël A, Wolf D, Bosset JF, Aletti P. Dosimetric consequences of breath-hold respiration in conformal radiotherapy of esophageal cancer. Phys Med. 2006;22:119-126. |
| 25. | Zhao KL, Liao Z, Bucci MK, Komaki R, Cox JD, Yu ZH, Zhang L, Mohan R, Dong L. Evaluation of respiratory-induced target motion for esophageal tumors at the gastroesophageal junction. Radiother Oncol. 2007;84:283-289. |
| 26. | Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, Lozach P, Ollier JC, Pavy JJ, Mercier M. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161-167. |
| 27. | Launois B, Delarue D, Campion JP, Kerbaol M. Preoperative radiotherapy for carcinoma of the esophagus. Surg Gynecol Obstet. 1981;153:690-692. |
| 28. | Lee HK, Vaporciyan AA, Cox JD, Tucker SL, Putnam JB Jr, Ajani JA, Liao Z, Swisher SG, Roth JA, Smythe WR. Postoperative pulmonary complications after preoperative chemoradiation for esophageal carcinoma: correlation with pulmonary dose-volume histogram parameters. Int J Radiat Oncol Biol Phys. 2003;57:1317-1322. |
| 29. | Wang M, Gu XZ, Yin WB, Huang GJ, Wang LJ, Zhang DW. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of esophageal carcinoma: report on 206 patients. Int J Radiat Oncol Biol Phys. 1989;16:325-327. |
| 30. | Wang SL, Liao Z, Vaporciyan AA, Tucker SL, Liu H, Wei X, Swisher S, Ajani JA, Cox JD, Komaki R. Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys. 2006;64:692-699. |
| 31. | Wales Cancer Trial Unit on behalf of NCRI Upper Gastrointestinal clinical studies group. SCOPE 1: Study of Chemoradiotherapy in Oesophageal cancer Plus or Minus Erbitus, 2007. Available from: http://www.wctu.org.uk/trial.php?trial=scope. |
| 32. | Twine CP, Roberts SA, Lewis WG, Dave BV, Rawlinson CE, Chan D, Robinson M, Crosby TD. Prognostic significance of endoluminal ultrasound-defined disease length and tumor volume (EDTV) for patients with the diagnosis of esophageal cancer. Surg Endosc. 2010;24:870-878. |
| 33. | Pedrazzani C, Bernini M, Giacopuzzi S, Pugliese R, Catalano F, Festini M, Rodella L, de Manzoni G. Evaluation of Siewert classification in gastro-esophageal junction adenocarcinoma: What is the role of endoscopic ultrasonography? J Surg Oncol. 2005;91:226-231. |
| 34. | Tai P, Van Dyk J, Yu E, Battista J, Stitt L, Coad T. Variability of target volume delineation in cervical esophageal cancer. Int J Radiat Oncol Biol Phys. 1998;42:277-288. |
| 35. | Tai P, Van Dyk J, Battista J, Yu E, Stitt L, Tonita J, Agboola O, Brierley J, Dar R, Leighton C. Improving the consistency in cervical esophageal target volume definition by special training. Int J Radiat Oncol Biol Phys. 2002;53:766-774. |
| 36. | Mamede M, El Fakhri G, Abreu-e-Lima P, Gandler W, Nosé V, Gerbaudo VH. Pre-operative estimation of esophageal tumor metabolic length in FDG-PET images with surgical pathology confirmation. Ann Nucl Med. 2007;21:553-562. |
| 37. | Vinjamuri S, Ray S. Added value of PET and PET-CT in oesophageal cancer: a review of current practice. Nucl Med Commun. 2008;29:4-10. |
| 38. | Wong WL, Chambers RJ. Role of PET/PET CT in the staging and restaging of thoracic oesophageal cancer and gastro-oesophageal cancer: a literature review. Abdom Imaging. 2008;33:183-190. |