Published online May 15, 2021. doi: 10.4251/wjgo.v13.i5.424
Peer-review started: January 6, 2021
First decision: January 25, 2021
Revised: February 11, 2021
Accepted: April 22, 2021
Article in press: April 22, 2021
Published online: May 15, 2021
Given the poor synthetic function of cirrhotic liver, successful resection for patients with hepatocellular carcinoma (HCC) necessitates the ability to achieve resections with tumor free margins.
To validate post hepatectomy liver failure score (PHLF), compare it to other established systems and to stratify risks in patients with cirrhosis who underwent curative liver resection for HCC.
Between December 2010 and January 2017, 120 patients underwent curative resection for HCC in patients with cirrhosis were included, the pre-operative, operative and post-operative factors were recorded to stratify patients' risks of decompensation, survival, and PHLF.
The preoperative model for end-stage liver disease (MELD) score [odds ratio (OR) = 2.7, 95%CI: 1.2-5.7, P = 0.013], tumor diameter (OR = 5.4, 95%CI: 2-14.8, P = 0.001) and duration of hospital stay (OR = 2.5, 95%CI: 1.5-4.2, P = 0.001) were significant independent predictors of hepatic decompensation after resection. While the preoperative MELD score [hazard ratio (HR) = 1.37, 95%CI: 1.16-1.62, P < 0.001] and different grades of PHLF (grade A: HR = 2.33, 95%CI: 0.59-9.24; Grade B: HR = 3.15, 95%CI: 1.11-8.95; Grade C: HR = 373.41, 95%CI: 66.23-2105.43; P < 0.001) and HCC recurrence (HR = 11.67, 95%CI: 4.19-32.52, P < 0.001) were significant independent predictors for survival.
Preoperative MELD score and tumor diameter can independently predict hepatic decompensation. While, preoperative MELD score, different grades of PHLF and HCC recurrence can precisely predict survival.
Core Tip: The present study clearly confirmed that, in patients with cirrhosis who underwent curative resection for hepatocellular carcinoma (HCC), the preoperative model for end-stage liver disease (MELD) score, tumor diameter and the duration of hospital stay were independent predictors of decompensation. While, preoperative MELD score, different grades of post hepatectomy liver failure score and HCC recurrence were independent predictors for survival.
- Citation: Elshaarawy O, Aman A, Zakaria HM, Zakareya T, Gomaa A, Elshimi E, Abdelsameea E. Outcomes of curative liver resection for hepatocellular carcinoma in patients with cirrhosis. World J Gastrointest Oncol 2021; 13(5): 424-439
- URL: https://www.wjgnet.com/1948-5204/full/v13/i5/424.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i5.424
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world; it represents more than 5% of all malignances[1]. In Egypt, HCC is one of the three frequently diagnosed cancers[2]. Unlike most of other solid tumors, the coexistence of cirrhosis is a life-threatening condition that complicates patients’ prognosis. The prognosis of patients is solely related to tumor stage and liver condition that determine the applicability and efficacy of therapy. Accordingly, the art of prognostication depends on four tightly closed aspects: The synthetic power of liver, stage of tumor at diagnosis, overall general condition of the patients and the efficacy and suitability of treatment modality[3-5].
Numerous staging systems and scores for HCC have been proposed to standardize HCC treatment protocols[6]. Among these staging systems, the most widely adopted systems are the barcelona clinic liver cancer (BCLC) and clip systems[7-9]. The aforementioned staging modalities rely on three factors guiding treatment decisions, namely: liver functions, tumor characteristics and the patients’ general condition.
Unfortunately, most patients with HCC in Egypt are initially diagnosed with intermediate or advanced stages. Therefore, the curative treatments including resection are limited only to a minority of patients. The surgical treatment for patients with HCC dictates subtle evaluation to assess resectability based on many considerations including anatomic considerations, liver synthetic function, and the patients’ overall general condition. The success of resection depends on the ability to achieve a resection with tumor free margins while leaving behind an adequate liver volume[6].
Moreover, the term of post hepatectomy liver failure (PHLF) has been introduced in 2011 by the International Study Group of Liver Surgery to describe the increase of international normalized ratio (INR) and serum bilirubin on or after postoperative day 5. It provides a grading of severity, based on the impact on the patients’ clinical management[10,11]. However, given the varied tumor and patient characteristics, it is difficult to identify the most accurate prognostic factors associated with improved survival and the predictors of decompensation after curative resection for HCC in patients with cirrhosis. So, we aimed to validate PHLF score and compare it with other established scoring systems as model for end-stage liver disease (MELD), BCLC, Milan criteria as well as to stratify risks in patients with cirrhosis who underwent curative liver resection for HCC.
Between January 2010 and December 2017, 920 elective liver resections were performed at National Liver Institute, Menoufia University, Egypt. After exclusion of patients with hepatic resections for causes other than HCC on top of liver cirrhosis, 120 patients were eligible for this retrospective study.
Inclusion criteria: We included patients with HCC on top of Child-Pugh class A cirrhosis, HCC diagnosis was based on triphasic computed tomography (CT) and/or dynamic magnetic resonance imaging (MRI) with the typical radiological hallmarks of HCC, i.e. contrast uptake in the arterial phase and washout in the venous/late phase. We excluded patients with liver tumors other than HCC, patients who underwent combined resection and radiofrequency ablation.
Pre-operative data: (1) demographic data including age and gender, the cause of liver cirrhosis, associated co-morbidities; (2) preoperative laboratory investigations: Complete blood count (CBC), serum total and direct bilirubin, aspartate aminotransferase, alanine aminotransferase (ALT), albumin, creatinin, INR alpha-fetoprotein (AFP) were measured using Cobas Integra 800 Auto analyzer (Roche Diagnostics Ltd., Mannheim, Germany); (3) preoperative diagnostic imaging: Pelvi-abdominal ultrasound with doppler study on the hepatic vasculature (was done one day before surgery), tri-phasic CT abdomen and pelvis and or MRI (was done within one month before surgery). Preoperative upper endoscopy for assessment of portal hypertension. was done; and (4) assessment of different stages and scores were done including Child-Pugh-Turcotte scoring system[12], MELD score[13], BCLC[9], Milan Criteria[14] and pre-operative assessments for surgical operation were done.
The operative data: Including type of operation: open or laparoscopic resection, type of resection and the amount of blood loss were reported.
Postoperative data: Including admission to intensive care units (ICU), plasma or blood transfusion. Liver function tests were done routinely on post-operative day 1, 3, 5, and 7. All complications were recorded prospectively. Early postoperative medical or surgical complications (according to the Clavien grades of postoperative complications)[15], and their management plan were recorded and classified. The postoperative complications were divided into major and minor. Minor complications were the adverse events with no or minimal impact on in-hospital stay. Life-threatening complications were considered as major postoperative complications.
On discharge: Reassessment of liver and renal function tests, CBC, INR and the patient’s clinical condition were assessed to calculate the Child-Pugh score, and to assess the general condition of the patient.
Follow-up: Measurement of serum AFP level and CT were done, one and three months after discharge. The predictors of hepatic decompensation at one and three months after resection were statistically assessed. Recurrence was diagnosed on the basis of HCC diagnosis.
Survival and mortality: Analysis of survival and the causes of death were conducted. The perioperative mortality was defined as the mortality during the first 30 d’ post-operative.
The primary endpoints of the study: Postoperative decompensation (defined as development of PHLF) and postoperative mortality, defined as death related to liver resection occurring during hospital stay or within 60 d of surgery to be determined.
Ethical approval: The study protocol was approved by the ethics committee (for medical research) in accordance with the Declaration of Helsinki and by the Institutional Review Board of the National Liver Institute, Menoufia University-Egypt, (IRB number IRB00003413) in February 2017. Written informed consent was obtained from each patient included in the study.
Data was collected and entered to the computer using SPSS program for statistical analysis. Data was entered as numerical or categorical, as appropriate. Quantitative data was shown as mean ± SD and qualitative data was expressed as frequency and percentages.
Survival was calculated using Kaplan-Meier curves. Chi Square test was used to measure the association between qualitative variables. Student t-test and Mann-Whitney test was used to compare means of 2 sets of quantitative normally and non-normally distributed data, respectively. Survival and decompensation were compared between different groups using the log-rank test for univariate analyses. Multivariate analyses were conducted with step-down Cox’s proportional hazard regression models to identify the predictors of mortality.
Predictors of hepatic decompensation, and recurrence were analyzed first by means of logistic regression test. We then performed multivariate analysis, including those variables with P < 0.05. Two-tailed P values were considered statistically significant if less than 0.05.
The preoperative demographics and characteristics were summarized in Table 1. Forty-two patients (35%) had gastroesophageal varices. Forty-seven patients (39.2%) received antiviral treatment for chronic hepatitis C virus (HCV) infection before surgery. Following surgery, blood and plasma transfusions were required in 12 (10%) and 6 (5%) patients, respectively. After resection, as shown in Table 2, patients had mean serum bilirubin and albumin of 1.5 ± 0.64 mg/dL, 3.2 ± 0.41 g/dL, respectively and a mean INR of 1.3 ± 0.1. Furthermore, patients had a mean Child-Pugh score of 6.62 ± 1.54 and 6.51 ± 1.1 upon discharge and 3 mo post-operatively, respectively. Postoperative MELD score was ≤ 10 in 68 patients (56.7%) with a mean score of 10.62 ± 2.27. PHLF developed in 44 (36.7%) patients; PHLF was classified as grade A, B and C in 16 (13.3%), 22 (18.3%) and 6 (5%) patients, respectively. Peri-operative mortality rate was 3.3%. Fifty patients (41.7%) died over a median follow-up of 34.4 mo (range: 1-64). Postoperative complications included hemorrhage, bile leak, wound infection and chest infection in 4 (3.3%), 2 (1.7%), 4 (3.3%) and 14 (11.7%) patients, respectively.
| mean ± SD/ n (%) | |
| Age in years (mean ± SD) | 59.23 ± 6.52 |
| HCV-related liver disease, n (%) | 105 (87.5) |
| Hypertensive, n (%) | 34 (28.3) |
| Diabetic, n (%) | 36 (30) |
| Previous abdominal operations, n (%) | 34 (28.3) |
| Child-Pugh class, n (%) | |
| A5 | 90 (75) |
| A6 | 30 (25) |
| MELD score (mean ± SD) | 8.54 ± 1.78 |
| MELD score, n (%) | |
| < 10 | 90 (75) |
| 10-20 | 30 (25) |
| BCLC stage, n (%) | |
| 0 | 12 (10) |
| A | 72 (60) |
| B | 36 (30) |
| Pre-operative upper endoscopy | |
| Oesophageal Varices, n (%) | |
| No | 78 (65) |
| Yes | 42 (35) |
| Pre-operative imaging | |
| Diameter of largest tumor in cm (mean ± SD) | 4.57 ± 2.03 |
| Tumor diameter > 5 cm, n (%) | |
| No | 86 (71.7) |
| Yes | 34 (28.3) |
| Serum Bilirubin (mg/dL) | 0.87 ± 0.34 |
| Aspartate Aminotransferase (U/L) | 53.2 ± 29.6 |
| Alanine Aminotransferase (U/L) | 44.32 ± 25.65 |
| Serum Albumin (g/dL) | 3.78 ± 0.48 |
| International normalized ratio | 1.16 ± 0.1 |
| Serum creatinine (mg/dL) | 0.86 ± 0.22 |
| Platelets count ( 103/mm3) | 141 ± 64.32 |
| Serum AFP (ng/mL) | 193.37 ± 343.37 |
| Serum AFP (ng/mL), n (%) | |
| < 20 | 52 (43.3) |
| 20-400 | 30 (25) |
| > 400 | 20 (16.7) |
| Undocumented | 18 (15) |
| Platelets < 150.000/mm3, n (%) | 64 (53.3) |
| Anatomical resection, n (%) | 22 (18.3) |
| Use of Harmonic scalpel, n (%) | 40 (33.3) |
| Use of Habib sealer, n (%) | 40 (33.3) |
| Use of CUSA, n (%) | 2 (1.7) |
| Intraoperative bleeding, n (%) | 4 (3.3) |
| Blood transfusion, n (%) | 12 (10) |
| Plasma transfusion, n (%) | 6 (5) |
| Grade of differentiation, n (%) | |
| 1 or 2 | 58 (48.3) |
| 3 or 4 | 38 (31.7) |
| Undocumented | 24 (20) |
| Lymph/vascular invasion, n (%) | 44 (36.7) |
| mean ± SD/ n (%) | |
| Serum bilirubin (mg/dL) | 1.5 ± 0.64 |
| AST (U/L) | 29.95 ± 11.31 |
| ALT (U/L) | 20.51 ± 10.03 |
| Serum albumin (g/dL) | 3.21 ± 0.41 |
| INR | 1.25 ± 0.14 |
| Serum Creatinin (mg/dL) | 0.87 ± 0.23 |
| Serum AFP (ng/mL) | 2109.7 ± 10795.17 |
| CRP (mg/L) | 45.99 ± 31.19 |
| Length of the hospital stay (d) | 8.9 ± 5.05 |
| ICU stay (d) | 4.28 ± 4.19 |
| Child-Pugh score | |
| On discharge | 6.62 ± 1.54 |
| 3-mo post-operative | 6.51 ± 1.1 |
| MELD score | 10.63 ± 2.27 |
| ICU stay, n (%) | 58 (48.3) |
| MELD score, n (%) | |
| ≤ 10 | 68 (56.7) |
| > 10 | 52 (43.3) |
| 50-50 criteria, n (%) | |
| Absent | 116 (96.7) |
| Present | 4 (3.3) |
| PHLF, n (%) | |
| Absent | 76 (63.3) |
| Grade A | 16 (13.3) |
| Grade B | 22 (18.3) |
| Grade C | 6 (5) |
| Post-operative complications, n (%) | |
| Hemorrhage | 4 (3.3) |
| Bile leak | 2 (1.7) |
| Wound infection | 4 (3.3) |
| Chest infection | 14 (11.7) |
| Recurrence of HCC, n (%) | 34 (28.3) |
| Post-operative decompensation, n (%) | 50 (41.7) |
| Peri-operative mortality, n (%) | 4 (3.3) |
| Overall survival, n (%) | |
| Alive | 70 (58.3) |
| Died | 50 (41.7) |
Univariate analyses identified preoperative factors associated with hepatic decompensation after resection in (Table 3). The lack of anti-viral therapy for HCV prior to surgery, advanced BCLC stage, and preoperative MELD scores between 10-20 were significantly associated with postoperative liver decompensation (P < 0.001). On univariate analyses, the tumor characteristics that were significantly associated with hepatic decompensation included multiple tumor foci (P = 0.027), tumors with diameters > 5 cm, extension beyond Milan criteria (P < 0.001), and presence of lymph vascular invasion. (P = 0.003) Operative factors significantly associated with hepatic decompensation after resection on univariate analysis included non-laparoscopic resection (P < 0.001) and long ICU admission (P = 0.004).
| No decompensation (70 patients) | Decompensation (50 patients) | P value | ||
| Age, n (%) | ||||
| < 60 yr | 36 (58.1) | 26 (41.9) | 0.004 | 0.951 |
| ≥ 60 yr | 34 (58.6) | 24 (41.0) | ||
| Antiviral therapy for HCV, n (%) | ||||
| No treatment | 34 (46.6) | 39 (53.4) | 29.143 | < 0.001c |
| DAAs | 34 (82.9) | 7 (17.1) | ||
| Interferon | 2 (33.3) | 4 (66.7) | ||
| Clinically significant portal hypertension, n (%) | ||||
| No | 52 (66.7) | 26 (33.3) | 7.7 | 0.006b |
| Yes | 17 (40.5) | 25 (59.5) | ||
| BCLC stage, n (%) | ||||
| 0 | 12 (100) | 0 (0) | 40.0 | < 0.001c |
| A | 52 (72.2) | 20 (27.8) | ||
| B | 6 (16.7) | 30 (83.3) | ||
| MELD score, n (%) | ||||
| < 10 | 60 (68.2) | 28 (31.8) | 22.721 | < 0.001c |
| 10-20 | 4 (15.4) | 22 (84.6) | ||
| Pre-operative Child-Pugh score, n (%) | ||||
| A5 | 54 (60) | 36 (40) | 0.411 | 0.521 |
| A6 | 16 (53.3) | 14 (46.7) | ||
| AFP (ng/mL), n (%) | ||||
| < 20 | 30 (57.7) | 22 (42.3) | 3.509 | 0.173 |
| 20-400 | 20 (66.7) | 10 (33.3) | ||
| > 400 | 8 (40) | 12 (60) | ||
| Pre-operative serum bilirubin (mg/dL), n (%) | ||||
| ≤ 1 | 46 (57.5) | 34 (42.5) | 0.201 | 0.654 |
| > 1 | 18 (52.9) | 16 (47.1) | ||
| Tumor diameter > 5 cm, n (%) | ||||
| No | 64 (74.4) | 22 (25.6) | 32.311 | < 0.001c |
| Yes | 6 (17.6) | 28 (82.4) | ||
| Number of tumors, n (%) | ||||
| Single | 68 (60.7) | 44 (39.3) | 5.869 | 0.027a |
| More than one | 0 (0) | 4 (100) | ||
| Site of tumor(s), n (%) | ||||
| Right lobe | 36 (62.1) | 22 (37.9) | 1.626 | 0.443 |
| Left lobe | 32 (57.1) | 24 (42.9) | ||
| Bilobar | 2 (100) | 0 (0) | ||
| Milan criteria, n (%) | ||||
| Within | 36 (69.2) | 16 (30.8) | 15.537 | < 0.001c |
| Beyond | 8 (25) | 24 (75) | ||
| Tumor differentiation, n (%) | ||||
| Grade 1 or 2 | 38 (63.3) | 22 (36.7) | 1.646 | 0.2 |
| Grade 3 or 4 | 18 (50) | 18 (50) | ||
| Lymph/vascular invasion, n (%) | ||||
| No | 38 (70.4) | 16 (29.6) | 8.593 | 0.003b |
| Yes | 18 (40.9) | 26 (59.1) | ||
| Type of resection, n (%) | ||||
| Open | 28 (42.4) | 38 (57.6) | 13.473 | < 0.001c |
| Laparoscopic | 12 (100) | 0 (0) | ||
| Anatomical resection, n (%) | ||||
| No | 38 (57.6) | 28 (42.4) | 0.978 | 0.323 |
| Yes | 10 (45.5) | 12 (54.5) | ||
| Extension of hepatectomy, n (%) | ||||
| One segment | 30 (51.7) | 28 (48.3) | 2.241 | 0.326 |
| Two segments | 8 (44.4) | 10 (55.6) | ||
| Three segments | 2 (100) | 0 (0) | ||
| ICU admission, n (%) | ||||
| No | 44 (71) | 18 (29) | 8.425 | 0.004b |
| Yes | 26 (44.8) | 32 (55.2) | ||
| Postoperative complications, n (%) | ||||
| Hemorrhage | 4 (100) | 0 (0) | 2.956 | 0.14 |
| Bile leak | 0 (0) | 2 (100) | 2.847 | 0.172 |
| Wound infection | 0 (0) | 4 (100) | 5.793 | 0.028a |
| Chest infection | 2 (14.3) | 12 (85.7) | 12.652 | < 0.001c |
| PHLF, n (%) | ||||
| Absent | 54 (71.1) | 22 (28.9) | 22.305 | < 0.001c |
| Grade A | 10 (62.5) | 6 (37.5) | ||
| Grade B | 6 (27.3) | 16 (72.7) | ||
| Grade C | 0 (0) | 6 (100) | ||
| 50-50 criteria, n (%) | ||||
| Absent | 70 (60.3) | 46 (39.7) | 5.793 | 0.028a |
| Present | 0 (0) | 4 (100) | ||
| HCC recurrence, n (%) | 16 (47.1) | 18 (52.9) | 2.481 | 0.115 |
| No decompensation | Decompensation | t-test | P value | |
| mean ± SD | ||||
| Post-operative bilirubin (mg/dL) | 1.21 ± 0.49 | 1.98 ± 0.57 | -7.353 | < 0.001c |
| Post-operative AFP (ng/mL) | 3020.12 ± 13277.79 | 371.63 ± 761.79 | 0.931 | 0.355 |
| Post-operative Child-Pugh score | 5.78 ± 0.65 | 7.62 ± 0.58 | -14.768 | < 0.001c |
| Age | 58.77 ± 6.68 | 59.88 ± 6.29 | -0.918 | 0.36 |
| Pre-operative Bilirubin (mg/dL) | 0.87 ± 0.31 | 0.87 ± 0.38 | 0.007 | 0.994 |
| Pre-operative MELD score | 8.03 ±1.44 | 9.2 ± 1.96 | -3.541 | 0.001b |
| Pre-operative Child score | 5.23 ± 0.42 | 5.28 ± 0.45 | -0.637 | 0.525 |
| Pre-operative AFP (ng/mL) | 149.58 ± 239.28 | 251.09 ± 441.64 | -1.379 | 0.173 |
| Tumor diameter (cm) | 3.77 ± 1.32 | 5.68 ± 2.33 | -5.238 | < 0.001c |
| ICU stay (d) | 2.92 ± 1.35 | 5.38 ± 5.29 | -2.522 | 0.016a |
| Hospital stay (d) | 6.86 ± 2.08 | 11.26 ± 6.34 | -4.092 | < 0.001c |
| Post-operative bilirubin (mg/dL) | 1.21 ± 0.49 | 1.98 ± 0.57 | -7.353 | < 0.001c |
| Post-operative AFP (ng/mL) | 3020.12 ± 13277.79 | 371.63 ± 761.79 | 0.931 | 0.355 |
| Post-operative Child-Pugh score | 5.78 ± 0.65 | 7.62 ± 0.58 | -14.768 | < 0.001c |
Post-operative factors that significantly associated with hepatic decompensation on univariate analyses included: longer ICU (P = 0.016) and hospital stays (P < 0.001), the development of wound infection and fulfillment of the 50-50 criteria (P = 0.028), chest infection, advanced grades of PHLF, higher postoperative bilirubin levels and higher Child-Pugh scores (P < 0.001) (Table 3).
In multivariate analysis, preoperative MELD score [odds ratio (OR) = 2.7, 95%CI: 1.2-5.7, P = 0.013], tumor diameter (OR = 5.4, 95%CI: 2-14.8, P = 0.001) and length of stay After radical resection of liver cancer, the length of hospital stay (OR = 2.5, 95%CI: 1.5-4.2, P = 0.001) is an important independent predictor of liver decompensation (Table 4).
| OR | 95%CI | P value | |
| Age | 1.09 | 0.96-1.25 | 0.184 |
| Preoperative MELD score | 2.65 | 1.23-5.72 | 0.013a |
| Preoperative serum bilirubin | 0.15 | 0.01-3.63 | 0.242 |
| Clinically significant portal hypertension | |||
| No | Reference | Reference | 0.191 |
| Yes | 4.39 | 0.48-40.25 | |
| Tumor diameter (cm) | 5.42 | 1.99-14.77 | 0.001b |
| Hospital stay (d) | 2.49 | 1.46-4.22 | 0.001b |
| ICU admission | |||
| No | Reference | Reference | 0.448 |
| Yes | 0.42 | 0.05-3.92 |
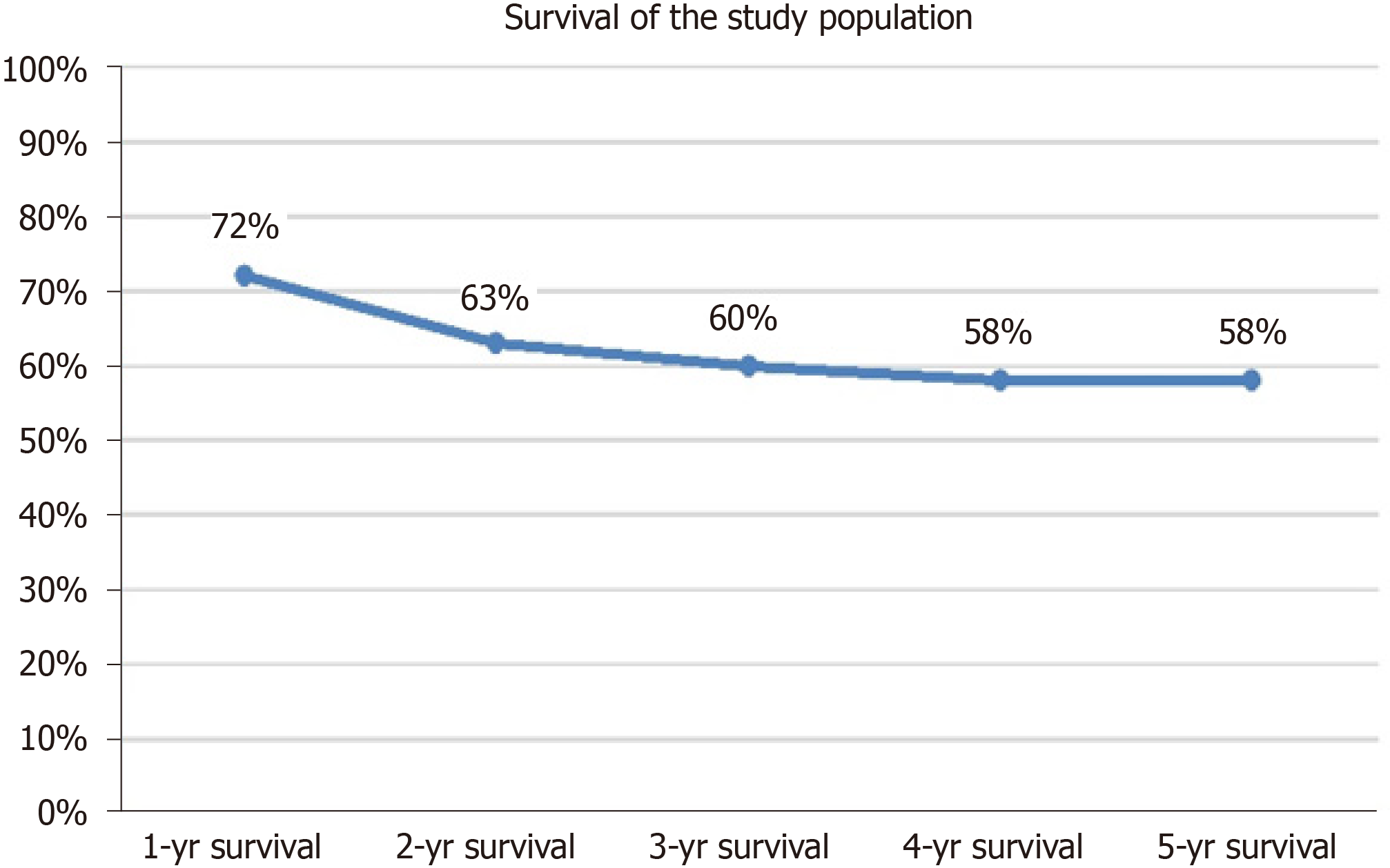
The survival of the study population was shown in Figure 1. The 1-year, 2-year, 3-year, 4-year, and 5-year survival rates after the liver resection were 72%, 63%. 60%, 58%, and 58% respectively. The perioperative mortality rate after liver resection (within 30-d after the operation) was 4/120 (3.3%), (Figure 1).
The identified prognostic factors of the survival after resection in univariate analysis were demonstrated in Table 5: The preoperative AFP levels > 400 ng/mL (P = 0.003), development of PHLF (P = 0.004) and its advanced grade (P < 0.001), pre-operative MELD scores between 10-20, advanced BCLC stage, fulfillment of the 50-50 criteria, postoperative MELD scores > 10, postoperative decompensation and HCC recurrence (P < 0.001).
| Mean survival time (mo) | 95%CI | Log-rank test | P value | |
| Clinically significant portal hypertension | ||||
| No | 40.6 | 35.3-46 | 0.551 | 0.458 |
| Yes | 39.5 | 30.9-48.1 | ||
| Preoperative AFP > 400 ng/mL | ||||
| No | 43.9 | 38.3-49.5 | 8.787 | 0.003b |
| Yes | 24.6 | 14.1-35.2 | ||
| Preoperative MELD score | ||||
| < 10 | 46.38 | 41.13-51.63 | 19.209 | < 0.001c |
| 10-20 | 22.55 | 13.44-31.65 | ||
| BCLC stage | ||||
| 0 | 51.9 | 40.5-63.2 | 22.328 | < 0.001c |
| A | 47.9 | 42.1-53.6 | ||
| B | 26.1 | 18.3-33.8 | ||
| PHLF | ||||
| No | 47.1 | 41.4-52.8 | 8.489 | 0.004b |
| Yes | 32.1 | 24.8-39.4 | ||
| PHLF grade | ||||
| Absent | 47.1 | 41.4-52.8 | 114.117 | < 0.001c |
| A | 42.2 | 30.4-53.9 | ||
| B | 31.7 | 23-40.4 | ||
| C | 2.1 | 0.5-3.6 | ||
| 50-50 criteria | ||||
| Absent | 43.6 | 38.8-48.3 | 156.853 | < 0.001c |
| Present | 0.8 | 0.7-0.8 | ||
| Post-operative MELD score | ||||
| ≤ 10 | 49.3 | 43.4-55.3 | 12.29 | < 0.001c |
| > 10 | 32.7 | 25.8-39.7 | ||
| Post-operative decompensation | ||||
| No | 52.2 | 47.1-57.4 | 27.716 | < 0.001c |
| Yes | 28.3 | 21.1-35.5 | ||
| HCC recurrence | ||||
| No | 53.3 | 48.7-57.9 | 69.639 | < 0.001c |
| Yes | 13.2 | 9.6-16.8 |
Multivariate Cox regression analysis was then performed to identify independent prognostic factors for survival, preoperative MELD score [hazard ratio (HR) = 1.37, 95% CI: 1.16-1.62, P < 0.001] and different levels of PHLF (Grade A: HR = 2.33, 95%CI: 0.59-9.24; Grade B: HR = 3.15, 95%CI: 1.11-8.95; Grade C: HR = 373.41, 95%CI: 66.23-2105.43; P < 0.001), postoperative HCC recurrence resection (HR = 11.67, 95%CI: 4.19-32.52, P < 0.001) (Table 6).
| HR | 95%CI | P value | |
| Age (yr) | 1.02 | 0.96-1.08 | 0.493 |
| Preoperative MELD score | 1.37 | 1.16-1.62 | < 0.001c |
| Preoperative AFP (ng/mL) | 1.001 | 1-1.002 | 0.08 |
| BCLC stage | |||
| 0 | Reference | Reference | 0.757 |
| A | 0.59 | 0.12-2.91 | |
| B | 0.45 | 0.06-3.65 | |
| PHLF grade | |||
| Absent | Reference | Reference | < 0.001c |
| A | 2.33 | 0.59-9.24 | |
| B | 3.15 | 1.11-8.95 | |
| C | 373.41 | 66.23-2105.43 | |
| Postoperative MELD score > 10 | 1.03 | 0.39-2.67 | 0.958 |
| Postoperative decompensation | 2.03 | 0.64-6.46 | 0.23 |
| HCC recurrence | 11.67 | 4.19-32.52 | < 0.001c |
Although the technical improvements in the field of liver surgery has increased the possibility to include more patients with different grades and severities of underlying liver cirrhosis, there is a potentiality of impaired postoperative recovery and favor of occurrence of post-operative liver cell failure[16-19]. In this context, it is crucial to incorporate patients’ preoperative status together with the extent of resection, the intraoperative course and the postoperative factors for the risk stratification and precise prediction of outcome after resection.
In the absence of standardized definitions of liver failure and its predictors, outside the context of surgery that can be easily applied in the early post-operative period in setting of HCC, many variables have been used to assess liver functions after curative liver resection in patients with HCC as ALT, gamma-glutamyl transferase, and alkaline phosphatase assessment, however, their results are influenced by the surgical insult and/or regeneration of the remnant liver rather than reflection of hepatic function[20].
Child-Pugh score, is a simple and attractive scoring system which was designed to predict the postoperative outcomes of cirrhotic patients in many settings[12]. However, it is likely to be biased in the postoperative period by inclusion of suggestive measures like degree of ascites and encephalopathy among its calculated variables in the early postoperative course. Moreover, hepatic encephalopathy and ascites were considered non- useful prognostic predictors of outcomes[21]. Similarly, the decrease of serum albumin, can also be induced by nonspecific factors such as nutritional status, postoperative ascites, and hem dilution[22]. In contrast to the above-mentioned variables, many authors have reported that prothrombin time and serum bilirubin, are less likely to be biased and can be used as a reflection of liver function[23,24]. However, the two variables have many threshold values and with different time points[23-26].
In our series, 50 patients developed decompensation within 3 mo post-operatively: In univariate analysis, the lack of antiviral therapy for HCV prior to surgery, presence of clinically significant portal hypertension (P = 0.006), advanced BCLC stage, preoperative MELD scores between 10-20, multiple tumor foci, tumors with diameters > 5 cm, extension beyond Milan criteria, and presence of lymph/vascular invasion were predictors of decompensation. Moreover, post-operative factors that significantly associated with hepatic decompensation after resection in univariate analysis included: Longer ICU and hospital stays the development of wound infection, chest infection, advanced grades of PHLF, higher postoperative bilirubin levels and higher Child-Pugh scores.
We have included the predictors of decompensation in our studied patients given its predictive value on patients’ survival, plus its role in compromising the patients’ quality of life. Moreover, liver decompensation increased the direct and indirect costs. Our findings in the currents study clearly revealed that the preoperative MELD score (P = 0.013), tumor diameter (P = 0.001) and the duration of hospital stay (P = 0.001) were independent predictors of hepatic decompensation after resection in multivariate analysis.
Many previous studies have evaluated the predictors of outcome after liver resection. Balzan et al[10] have evaluated the 50-50 criteria in patients with hepatectomy in a large number of patients, the authors included the association of 50% of Prothrombin time and the increase of serum bilirubin > 50 mL/L on the post -operative day 5- (the 50-50 criteria). They concluded that the 50-50 criteria is a simple and accurate predictor of more than 50% mortality rate after hepatectomy. The advantage of this criteria could be identified early enough, as a predictive of clinical evidence of complications and could be applied as a real time point even with limited health resources. This goes in line with our findings even with the application of 50-50 criteria in our patients with special characteristics' (curative resection for HCC in patients with cirrhosis).
Similarly, Rahbari et al[12] have assessed 3 clinical risk scores, namely (MELD score, the ‘‘50–50 criteria,’’ and the PHLF as clinical risk scores. The authors analyzed the aforementioned scores for morbidity and mortality in multivariate logistic regression analyses. The postoperative clinical risk scores are associated independently with outcome after hepatic resection. Based on their finding, the MELD score was an independent predictive factor that could be recommended for early prediction of overall morbidity, whereas the MELD score and the PHLF enabled adequate risk stratification regarding perioperative mortality. However, the 50–50 criteria on postoperative day 5 had low sensitivity (missed 74% of perioperative deaths) as independent predictor of mortality after hepatic resection.
The MELD score was originally introduced in early years of this century to predict short-term survival in patients undergoing transcutaneous intrahepatic porto-systemic shunt procedures[13], and it has been shown to be reliable and predictive in many settings[27-29]. It is widely accepted as a useful tool in assessing prognosis in patients with alcoholic hepatitis, liver transplantation according to guidelines adopted by the United Network for Organ Sharing in 2002[30].
Interestingly, MELD score seems to predict patient and graft survival especially in cadaveric liver transplantation. In this context, Schroeder et al[31] have evaluated MELD scores in cohort of patient underwent liver resection for different etiologies, in this study, MELD score failed to predict outcomes of the studied patients.
This study has several limitations that are inherent in retrospective cohorts. Firstly, the clinical picture obtained due to possible errors in the coding of primary diagnoses and treatment modalities. Moreover, we noted that, some of the studied patients had been treated with direct-acting antiviral agents s, with missed data in the rest of the patients. Furthermore, the complications associated with hospital resource utilization and mortality rate were not accurately assessed, which in turn limited the validity of the predictions. The 50-50 criteria were accomplished only in small number of patients according to our inclusion criteria. Finally, the patient quality of life and indirect costs incurred after discharge were not assessed. However, the aforementioned limitations are unlikely to compromise the results given the robust magnitude of the effects and the statistical significance of the observed effects in this study.
Given the findings of our study, it has many strengths as it is the first report in evaluating the PHLF in the setting of curative resection for HCC in patients with cirrhosis. It additionally evaluated homogenous group of cohorts; patient with cirrhosis and HCC who underwent curative resections, most of the studied cohort have HCV and hepatitis B virus related cirrhosis, we evaluated many prognostic predictors in one study and finally we have evaluated the possible variable (pre-operative, operative and post-operative variable) that might predict outcomes. However, there is a need for a possible scoring system to include all of these factors in simple score to give more validity for the prediction of decompensation and mortality in patients with HCC undergo curative resection on background of liver cirrhosis. In addition, the quality of life and cost should be accurately assessed.
Our study confirmed that, in patients with cirrhosis who underwent curative resection for HCC, the preoperative MELD score, tumor diameter and the duration of hospital stay can independently predict hepatic decompensation. While, preoperative MELD score, different grades of PHLF and HCC recurrence can precisely predict survival of patients with cirrhosis undergoing liver resection for HCC.
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world; and it is one of the three frequently diagnosed cancers in Egypt. Unfortunately, most patients with HCC in Egypt are initially diagnosed with intermediate or advanced stages. Therefore, the curative treatments including liver resection are limited only to a small percent of patients. The success of resection depends on the ability to achieve a resection with tumor free margins while leaving behind an adequate liver volume.
It is difficult to identify the most accurate prognostic factors associated with improved survival and the predictors of decompensation after curative resection.
To determine prognostic factors for survival and outcome after liver resection as well as validating post hepatectomy liver failure score (PHLF) and compare it to the performance of other established scoring systems which could help the prognosis of those patients after surgery.
We accrued data of 120 patients who had liver resection from 2010 to 2017 and included those with full follow up data. We performed analysis for the data to determine the prognostic factors and test the validity of the proposed score as well as compare it's validity to other established scoring systems.
Preoperative model of end stage liver disease (MELD) score and tumor diameter can precisely predict the risk of hepatic decompensation after surgery while preoperative MELD score together with different grades of PHLF and the incidence of HCC recurrence can predict survival of patients post operation.
The proposed (PHLF) scoring system as well as the established MELD score are good prognostic tools for survival while MELD score with tumor diameter are predictive for the risk of hepatic decompensation.
These models should be prospectively validated in determining decisions regarding hepatic resection in such group of patients.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang L S-Editor: Fan JR L-Editor: A P-Editor: Li JH
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25182] [Article Influence: 1937.1] [Reference Citation Analysis (6)] |
| 2. | Abdel-Wahab M, el-Enein AA, Abou-Zeid M, el-Fiky A, Abdallah T, Fawzy M, Fouad A, Sultan A, Fathy O, el-Ebidy G, elghawalby N, Ezzat F. Hepatocellular carcinoma in Mansoura-Egypt: experience of 385 patients at a single center. Hepatogastroenterology. 2000;47:663-668. [PubMed] [Cited in This Article: ] |
| 3. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ; Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1232] [Cited by in F6Publishing: 1298] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 4. | Mukozu T, Nagai H, Matsui D, Kanekawa T, Sumino Y. Serum VEGF as a tumor marker in patients with HCV-related liver cirrhosis and hepatocellular carcinoma. Anticancer Res. 2013;33:1013-1021. [PubMed] [Cited in This Article: ] |
| 5. | Elshimi E, Sakr MAM, Morad WS, Mohammad L. Optimizing the Diagnostic Role of Alpha-Fetoprotein and Abdominal Ultrasound by Adding Overexpressed Blood mRNA Matrix Metalloproteinase-12 for Diagnosis of HCV-Related Hepatocellular Carcinoma. Gastrointest Tumors. 2019;5:100-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1024] [Cited by in F6Publishing: 1165] [Article Influence: 145.6] [Reference Citation Analysis (1)] |
| 7. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2645] [Cited by in F6Publishing: 2727] [Article Influence: 109.1] [Reference Citation Analysis (0)] |
| 8. | Llovet JM, Fuster J, Bruix J; Barcelona-Clínic Liver Cancer Group. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl 2004; 10: S115-S120. . [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 500] [Cited by in F6Publishing: 500] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 9. | Selby LK, Tay RX, Woon WW, Low JK, Bei W, Shelat VG, Pang TC, Junnarkar SP. Validity of the Barcelona Clinic Liver Cancer and Hong Kong Liver Cancer staging systems for hepatocellular carcinoma in Singapore. J Hepatobiliary Pancreat Sci. 2017;24:143-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 789] [Cited by in F6Publishing: 767] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 11. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1224] [Cited by in F6Publishing: 1480] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 12. | Rahbari NN, Reissfelder C, Koch M, Elbers H, Striebel F, Büchler MW, Weitz J. The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. Ann Surg Oncol. 2011;18:3640-3649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Child CG II, Turcotte JG. Surgery and portal hypertension. In: Child CG III, editors. The liver and portal hypertension. Philadelphia, PA: Saunders, 1964: 10. [Cited in This Article: ] |
| 14. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1967] [Cited by in F6Publishing: 1911] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 15. | Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44-S57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 399] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 16. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6210] [Cited by in F6Publishing: 7316] [Article Influence: 487.7] [Reference Citation Analysis (0)] |
| 17. | Huo TI, Lui WY, Wu JC, Huang YH, King KL, Loong CC, Lee PC, Chang FY, Lee SD. Deterioration of hepatic functional reserve in patients with hepatocellular carcinoma after resection: incidence, risk factors, and association with intrahepatic tumor recurrence. World J Surg. 2004;28:258-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 800] [Cited by in F6Publishing: 778] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 19. | Ong GB, Lee NW. Hepatic resection. Br J Surg. 1975;62:421-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Rahbari NN, Koch M, Zimmermann JB, Elbers H, Bruckner T, Contin P, Reissfelder C, Schmidt T, Weigand MA, Martin E, Büchler MW, Weitz J. Infrahepatic inferior vena cava clamping for reduction of central venous pressure and blood loss during hepatic resection: a randomized controlled trial. Ann Surg. 2011;253:1102-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Reissfelder C, Rahbari NN, Koch M, Ulrich A, Pfeilschifter I, Waltert A, Müller SA, Schemmer P, Büchler MW, Weitz J. Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:3279-3288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Rahbari NN, Wente MN, Schemmer P, Diener MK, Hoffmann K, Motschall E, Schmidt J, Weitz J, Büchler MW. Systematic review and meta-analysis of the effect of portal triad clamping on outcome after hepatic resection. Br J Surg. 2008;95:424-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Rahbari NN, Koch M, Schmidt T, Motschall E, Bruckner T, Weidmann K, Mehrabi A, Büchler MW, Weitz J. Meta-analysis of the clamp-crushing technique for transection of the parenchyma in elective hepatic resection: back to where we started? Ann Surg Oncol. 2009;16:630-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406; discussion 406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 382] [Reference Citation Analysis (0)] |
| 25. | Jalan R, Sen S, Williams R. Prospects for extracorporeal liver support. Gut. 2004;53:890-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, Emile JF, Bismuth H. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Angermayr B, Cejna M, Karnel F, Gschwantler M, Koenig F, Pidlich J, Mendel H, Pichler L, Wichlas M, Kreil A, Schmid M, Ferlitsch A, Lipinski E, Brunner H, Lammer J, Ferenci P, Gangl A, Peck-Radosavljevic M. Child-Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut. 2003;52:879-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 223] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Erratum for the Report "Global distribution of earthworm diversity" by H. R. P. Phillips, C. A. Guerra, M. L. C. Bartz, M. J. I. Briones, G. Brown, T. W. Crowther, O. Ferlian, K. B. Gongalsky, J. van den Hoogen, J. Krebs, A. Orgiazzi, D. Routh, B. Schwarz, E. M. Bach, J. Bennett, U. Brose, T. Decaëns, B. König-Ries, M. Loreau, J. Mathieu, C. Mulder, W. H. van der Putten, K. S. Ramirez, M. C. Rillig, D. Russell, M. Rutgers, M. P. Thakur, F. T. de Vries, D. H. Wall, D. A. Wardle, M. Arai, F. O. Ayuke, G. H. Baker, R. Beauséjour, J. C. Bedano, K. Birkhofer, E. Blanchart, B. Blossey, T. Bolger, R. L. Bradley, M. A. Callaham, Y. Capowiez, M. E. Caulfield, A. Choi, F. V. Crotty, A. Dávalos, D. J. Diaz Cosin, A. Dominguez, A. E. Duhour, N. van Eekeren, C. Emmerling, L. B. Falco, R. Fernández, S. J. Fonte, C. Fragoso, A. L. C. Franco, M. Fugère, A. T. Fusilero, S. Gholami, M. J. Gundale, M. Gutiérrez López, D. K. Hackenberger, L. M. Hernández, T. Hishi, A. R. Holdsworth, M. Holmstrup, K. N. Hopfensperger, E. Huerta Lwanga, V. Huhta, T. T. Hurisso, B. V. Iannone III, M. Iordache, M. Joschko, N. Kaneko, R. Kanianska, A. M. Keith, C. A. Kelly, M. L. Kernecker, J. Klaminder, A. W. Koné, Y. Kooch, S. T. Kukkonen, H. Lalthanzara, D. R. Lammel, I. M. Lebedev, Y. Li, J. B. Jesus Lidon, N. K. Lincoln, S. R. Loss, R. Marichal, R. Matula, J. H. Moos, G. Moreno, A. Morón-Ríos, B. Muys, J. Neirynck, L. Norgrove, M. Novo, V. Nuutinen, V. Nuzzo, M. Rahman P, J. Pansu, S. Paudel, G. Pérès, L. Pérez-Camacho, R. Piñeiro, J.-F. Ponge, M. I. Rashid, S. Rebollo, J. Rodeiro-Iglesias, M. Á. Rodríguez, A. M. Roth, G. X. Rousseau, A. Rozen, E. Sayad, L. van Schaik, B. C. Scharenbroch, M. Schirrmann, O. Schmidt, B. Schröder, J. Seeber, M. P. Shashkov, J. Singh, S. M. Smith, M. Steinwandter, J. A. Talavera, D. Trigo, J. Tsukamoto, A. W. de Valença, S. J. Vanek, I. Virto, A. A. Wackett, M. W. Warren, N. H. Wehr, J. K. Whalen, M. B. Wironen, V. Wolters, I. V. Zenkova, W. Zhang, E. K. Cameron, N. Eisenhauer. Science. 2020;369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Salerno F, Merli M, Cazzaniga M, Valeriano V, Rossi P, Lovaria A, Meregaglia D, Nicolini A, Lubatti L, Riggio O. MELD score is better than Child-Pugh score in predicting 3-month survival of patients undergoing transjugular intrahepatic portosystemic shunt. J Hepatol. 2002;36:494-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 202] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 30. | Freeman RB Jr, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, Merion R, Wolfe R, Turcotte J, Teperman L; UNOS/OPTN Liver Disease Severity Score; UNOS/OPTN Liver and Intestine; and UNOS/OPTN Pediatric Transplantation Committees. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 501] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 31. | Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243:373-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |