Published online Oct 15, 2019. doi: 10.4251/wjgo.v11.i10.804
Peer-review started: April 22, 2019
First decision: June 4, 2019
Revised: July 11, 2019
Accepted: September 4, 2019
Article in press: September 5, 2019
Published online: October 15, 2019
Gastric cancer (GC) is a complex disease linked to a series of environmental factors and unhealthy lifestyle habits, and especially to genetic alterations. GC represents the second leading cause of cancer-related deaths worldwide. Its onset is subtle, and the majority of patients are diagnosed once the cancer is already advanced. In recent years, there have been innovations in the management of advanced GC including the introduction of new classifications based on its molecular characteristics. Thanks to new technologies such as next-generation sequencing and microarray, the Cancer Genome Atlas and Asian Cancer Research Group classifications have also paved the way for precision medicine in GC, making it possible to integrate diagnostic and therapeutic methods. Among the objectives of the subdivision of GC into subtypes is to select patients in whom molecular targeted drugs can achieve the best results; many lines of research have been initiated to this end. After phase III clinical trials, trastuzumab, anti-Erb-B2 receptor tyrosine kinase 2 (commonly known as ERBB2) and ramucirumab, anti-vascular endothelial growth factor receptor 2 (commonly known as VEGFR2) monoclonal antibodies, were approved and introduced into first- and second-line therapies for patients with advanced/metastatic GC. However, the heterogeneity of this neoplasia makes the practical application of such approaches difficult. Unfortunately, scientific progress has not been matched by progress in clinical practice in terms of significant improvements in prognosis. Survival continues to be low in contrast to the reduction in deaths from many common cancers such as colorectal, lung, breast, and prostate cancers. Although several target molecules have been identified on which targeted drugs can act and novel products have been introduced into experimental therapeutic protocols, the overall approach to treating advanced stage GC has not substantially changed. Currently, surgical resection with adjuvant or neoadjuvant radiotherapy and chemotherapy are the most effective treatments for this disease. Future research should not underestimate the heterogeneity of GC when developing diagnostic and therapeutic strategies aimed toward improving patient survival.
Core tip: The onset of gastric cancer is linked to genetic alterations, and environmental and lifestyle factors. Recent classifications (TCGA, ACRG) based on genetic alterations have shown significant neoplasia heterogeneity, which makes the practical application of such approaches more difficult. Numerous studies have been conducted on new specific targeted therapies in advanced gastric cancer in the field of precision medicine. The results have not been satisfactory in terms of survival, so elective therapy remains surgery associated with adjuvant and neoadjuvant chemotherapy. Future research should not underestimate the heterogeneity of gastric cancer when developing diagnostic and therapeutic strategies aimed toward improving patient survival.
- Citation: Bonelli P, Borrelli A, Tuccillo FM, Silvestro L, Palaia R, Buonaguro FM. Precision medicine in gastric cancer. World J Gastrointest Oncol 2019; 11(10): 804-829
- URL: https://www.wjgnet.com/1948-5204/full/v11/i10/804.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i10.804
Gastric cancer (GC) is a complex disease whose onset is linked to a series of genetic and environmental factors such as smoking and a high salt diet. Helicobacter pylori (H. pylori) is considered one of the most significant risk factors of GC. It is present in more than 70% of non-cardia GC cases and 90% of chronic gastritis cases[1], and its presence increases the risk of cancer (as compared to uninfected individuals)[2,3]. Recent research has shown that there is a correlation between the risk of GC and the characteristics of specific strains of H. pylori[4]. Moreover, H. pylori infection has been demonstrated to be essential for promoting chronic inflammation of the gastric epithelium and histological changes that sequentially lead to GC[5]. In this process, genetic and epigenetic alterations occur such as hypermethylation of DNA or mutations in genes including APC, WNT signaling pathway regulator (APC), tumor protein p53 (TP53), and KRAS proto-oncogene, GTPase (KRAS)[6].
Regarding treatment options, surgical resection with adjuvant or neoadjuvant radiotherapy and chemotherapy with cisplatin, 5-fluorouracil (5-FU), taxane, or irinotecan are the most effective treatments for GC. However, despite the increasing knowledge and progress in drug development, this disease has a very poor prognosis due to late diagnosis and extreme intra- and inter-tumor heterogeneity. The heterogeneity makes the choice of therapy difficult, emphasizing the need for both new indicators for patient classification and novel therapies capable of addressing genetic, molecular, and cellular heterogeneity within tumors. This review highlights the progress achieved in the molecular characterization of GC and how it has impacted diagnosis, prognosis, and therapy in clinical practice.
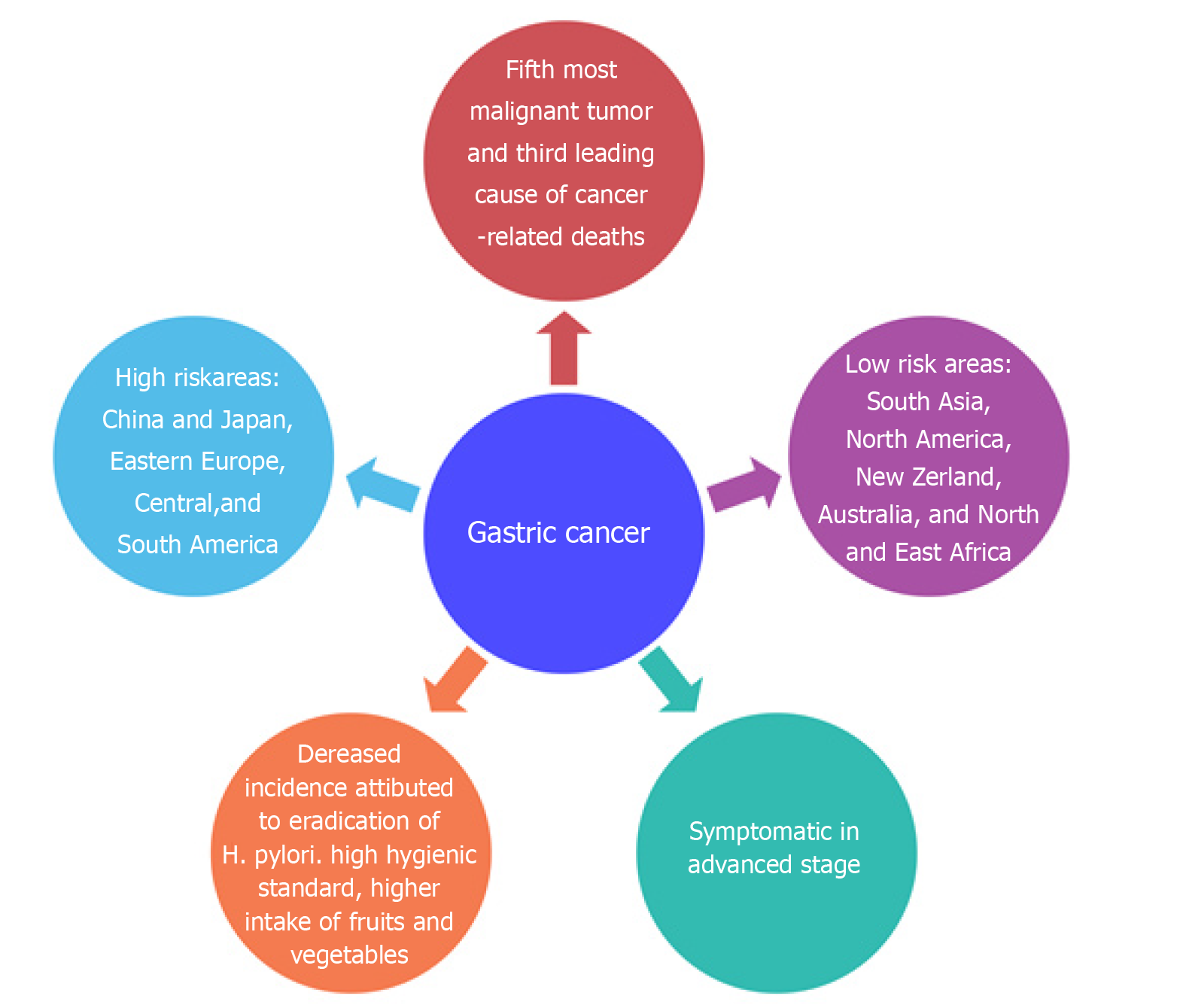
GC is the fifth most malignant tumor in worldwide and the third leading cause of cancer-related deaths[7]. Unfortunately, the disease becomes symptomatic in the advanced stage; thus, the 5-year survival rate is only high (90%) in Japan where diagnosis and early tumor resection are done[8]. In European countries, however, the survival rate is low, varying between 10% and 30%[9]. The incidence of GC has geographical variation, with more than 50% of new cases occurring in developing countries. The areas most at risk are represented by China and Japan, Eastern Europe, Central, and South America, while the areas with lowest risk are South Asia, North America, New Zealand, Australia, and North and East Africa[10]. In recent decades, a decrease in the incidence rate has been observed, especially in young patients with non-cardia, sporadic, and intestinal GC[11,12]. The decreased incidence of GC can be attributed to the better preservation of foods, higher hygienic standards, higher intake of fruits and vegetables, and the eradication of H. pylori[13]. Figure 1 summarizes the epidemiology of GC.
According to World Health Organization (commonly known as WHO) guidelines, GC can be classified as adenocarcinoma, ring-cell carcinoma, and undifferentiated carcinoma[14]. The Lauren's classification, which is widely used, classifies GC into intestinal, diffuse, and mixed/unclassified types based on macroscopic and microscopic differences[15]. It has been hypothesized that intestinal GC is associated with chronic atrophic gastritis and intestinal metaplasia, whereas the diffuse type originates from normal gastric mucosa. In European countries, the intestinal type is currently the most common GC[16-20]. It tends to occur more often in the distal part of the stomach, in high-risk areas and is often preceded by long-standing precancerous lesions[17]. On the other hand, the diffuse type is predominant among young patients. However, Lauren’s classification has a couple of key flaws. First, a large group of carcinomas do not fall into the two main types of carcinomas, intestinal or diffuse. This group of “unclassified” or “undetermined” gastric carcinomas include undifferentiated carcinomas and carcinomas that have dual differentiation (mixed intestinal and diffuse carcinomas). Second, there has been confusion regarding the “intestinal” term. Therefore, a change to Lauren’s classification has been proposed in which GCs are classified into four subtypes: Glandular, solid, isolated cell type, and mixed carcinoma[21].
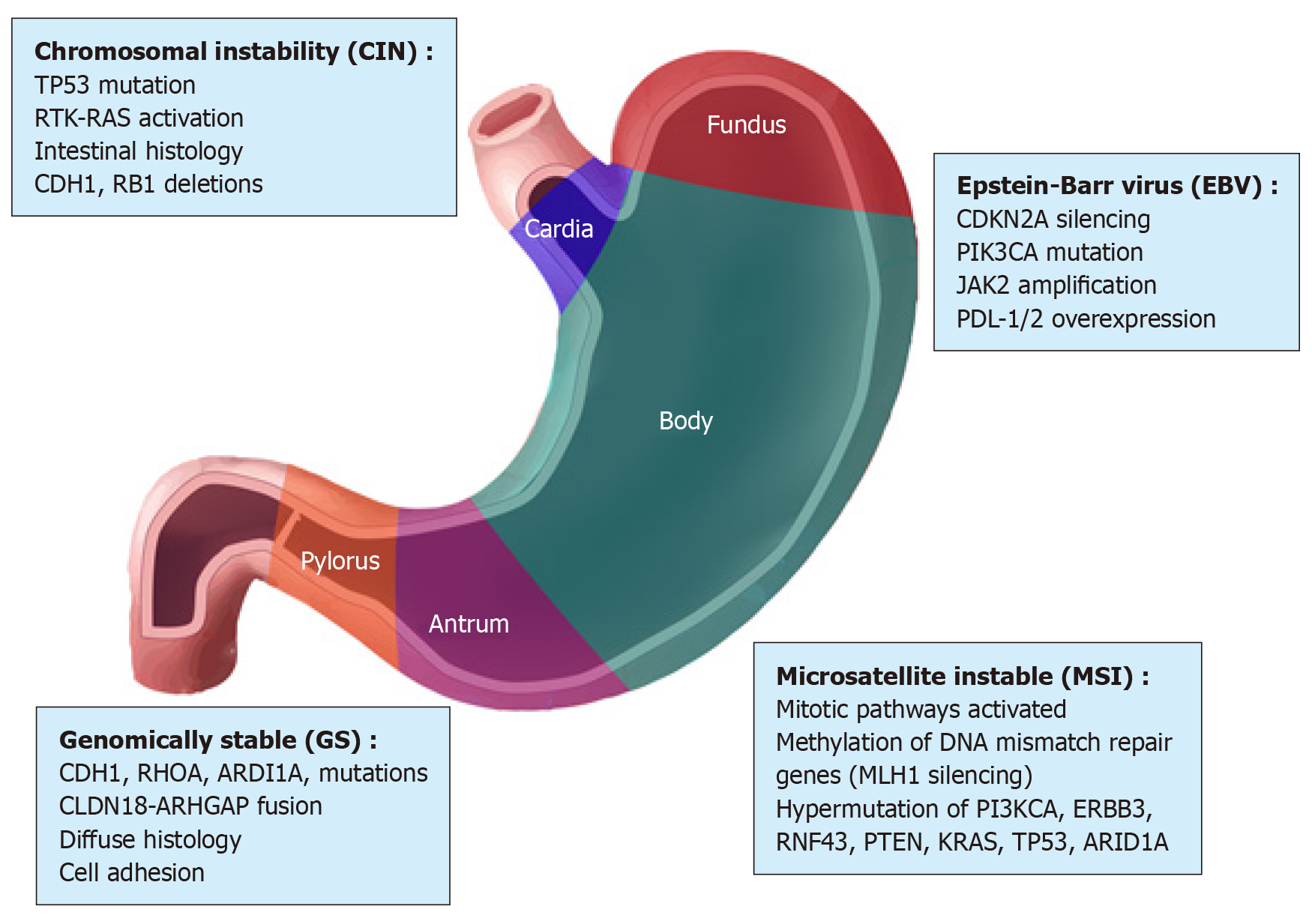
Advances in next-generation sequencing (NGS) and microarray technologies and a better understanding of cancer biology have provided opportunities to characterize the genome of tumors including GC. The molecular profile of the GC has enabled The Cancer Genome Atlas (TCGA) and the Asian Cancer Research Group (ACRG) to classify GC into subtypes. The new molecular classification of GC is complementary to the subtyping classification based on histopathological characteristics. It is important to note that the molecular classification of GC helps to identify the molecular alterations that may be targeted by therapy. Furthermore, the molecular profiles of GCs obtained from individual patients have offered new opportunities to identify biomarkers that can be predictive of the tumor response to treatment[22-24] and to guide the selection of cytotoxic drugs and targeted therapies. TCGA and ACRG classifications of GC should facilitate the development of personalized prognosis and treatment, as well as better patient stratification for the design of clinical trials. The molecular characterization of GC from TCGA has used different platforms, including exome sequencing, DNA copy number analysis, DNA methylation, mRNA and microRNA (miRNA) expression. It divides GC into four subtypes: Epstein-Barr virus (EBV)-positive, microsatellite instable (MSI), chromosomal instability (CIN), and genomically stable (GS) (Figure 2). Each of these GC subtypes is characterized by distinct features that provide prognostic information and suggest the potential benefits of targeted therapy.
EBV-positive tumors have mainly been found in the fundus and gastric body[25,26], and represent about 9% of GC cases. High DNA hypermethylation has been demonstrated in EBV-positive tumors, particularly of the cyclin-dependent kinase inhibitor 2A (commonly known as CDKN2A) promoter[27]. An estimated 80% of EBV-positive subtype tumors contain mutations in phosphatidylinositol 3-kinase CA (PIK3CA)[28] and amplification of Janus Kinase 2 (JAK2), CD274 molecule, and programmed cell death 1 ligand 2 (PDCD1LG2), which encode for respectively tyrosine kinase receptors, PD-L1 and PD-L2[29]. Based on these results, JAK2 inhibitors and PD-L1/2 antagonists should be explored as treatment options for EBV-positive tumors. Promising initial results have been reported with pembrolizumab, a humanized monoclonal antibody against programmed cell death 1 (PD-1)[30,31]. In addition to PIK3CA mutations, EBV-positive tumors have more recurrent AT-rich interaction domain 1A (ARID1A) (55%) and BCL6 corepressor (commonly known as BCOR) (23%) mutations[29,32], whereas only rare TP53 mutations have been observed.
Patients with MSI subtype generally have intestinal tumors, which are diagnosed in old age. MSI tumors (21.7% of GC cases) are characterized by genomic instability due to methylation of DNA mismatch repair genes including MutL homolog 1 (MHL1) and to a high incidence of mutations in PIK3CA[33], Erb-B2 receptor tyrosine kinase 3 (ERBB3)[34], ring finger protein 43 (RNF43)[35], phosphatase and tensin homolog (PTEN)[36], TP53[37], KRAS[38], and ARID1A[32] genes. Increased expression of components of the mitotic pathway such as the E2F transcription factor (E2F), aurora kinase A (AURKA), polo-like kinase 1 (PLK1), and forkhead box M1 (FOXM1) has been described in MSI tumors[29].
GS tumors (19.7%) are mainly diffuse and are diagnosed in younger patients[39]. GS tumors, which lack chromosomal alteration or MSI, exhibit the high expression of molecules involved in cell adhesion and pathways related to angiogenesis. They also have low mutation rates and ARID1A, ras homolog family member A (RHOA), and cadherin 1 (CDH1) are the most frequently mutated genes[40]. Previous studies have shown the loss of CDH1, which encodes the E-cadherin cell adhesion molecule, in hereditary diffuse GC[41]. TCGA data have also revealed the fusion of claudin 18-Rho GTPase activating protein 6 (CLDN18-ARHGAP6) or claudin 18-Rho GTPase activating protein 26 (CLDN18-ARHGAP26) and recurrent mutations in RHOA. CLDN18 and ARHGAP6 are respectively involved in the intercellular structure of the tight junction and the activation of Rho signaling (a signaling pathway in which intracellular and extracellular stimuli activate GTPase Rho), whereas RHOA modulates programmed cell death and contractility and motility of actomyosin-dependent cells[42-44]. Therefore, alterations in RHOA or CLDN18-ARHGAP6 could contribute to the lack of cell cohesion, dispersed growth, and programmed cell death resistance.
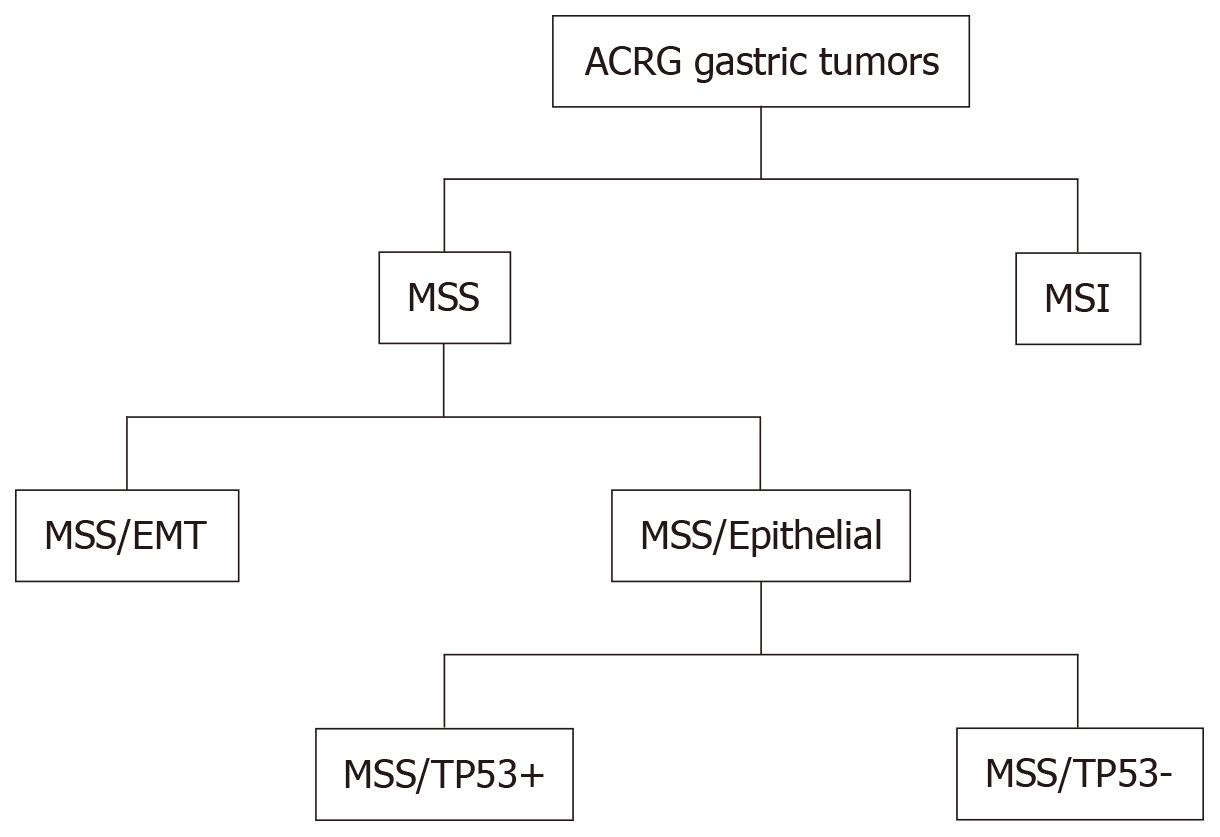
CIN tumors represent almost half of GC cases (49.8%), are mainly intestinal, and are most frequent in the cardia-gastro-esophageal junction. Chromosomal deletions affecting CDH1, catenin alpha 1 (CTNNA1) and RB transcriptional corepressor 1 (RB1) and mutations in TP53 (71%) are frequent in these tumors. CIN tumors present with amplification of genes encoding tyrosine kinase receptors such as epidermal growth factor receptor (EGFR), ERBB2, ERBB3, fibroblast growth factor receptor 2 (FGFR2), and MET proto-oncogene, receptor tyrosine kinase (MET); some transcription factors including the MYC proto-oncogene, basic helix-loop-helix transcription factor (MYC) and GATA binding protein 4 (GATA4); cell cycle regulators such as cyclin-dependent kinase 6 (CDK6), cyclin E1 (CCNE1), and cyclin D1 (CCND1) and other genes such as PDCD1LG2 and PIK3CA[29]. Alterations of these genes have been observed in advanced/metastatic GC[24]. By contrast, the ACRG analyzed samples from 300 Korean patients, classifying GC based on particular genetic signatures such as the activation status of TP53 and the MSI condition[45]. Four molecular subtypes have been identified: MSI, microsatellite stable (MSS) with active TP53 (MSS/TP53+), MSS with inactive TP53 (MSS/TP53-), and MSS with epithelial-mesenchymal transition (EMT) signature (MSS/EMT) (Figure 3).
These subtypes are associated with survival and recurrence. The MSI subtype has a better prognosis and a lower tendency to relapse. The MSS/TP53+ and MSS/TP53- subtypes have an intermediate prognosis, whereas the MSS/EMT subtype is associated with a high rate of recurrence and a lower survival rate. Moreover, MSI tumors are diagnosed at an early stage (I/II), and about 60% are intestinal and show a high frequency of mutations of PIK3CA, KRAS, ARID1A, and ALK receptor tyrosine kinase (ALK) genes; they also show loss of MLH1. Tumors of the MSS/TP53+ subtype include many EBV-positive cases compared to the other subtypes, and have a high prevalence of mutations in the APC, KRAS, PIK3CA, ARID1A, and SMAD family member 4 (SMAD4) genes[46] compared to the MSS/TP53- subtype. They also present amplification of the CCNE1 gene. The MSS/TP53- subtype is mainly Lauren intestinal and has TP53 mutations, with a low frequency of mutations affecting the other genes. This subtype also has amplification of EGFR, MYC, ERBB2, and CCNE1 genes. The MSS/EMT subtype predominantly consists of Lauren diffuse tumors, and tend to be diagnosed at a younger age. This subtype has low cell adhesion due to loss of CDH1 and has the least number of mutations. ARID1A is among the most frequently mutated gene. The ACRG classification is also applicable to other large independent cohorts[45]. The differences between the two classifications (TGCA and ACRG) reflect the different approaches and platforms used, and the ethnicity of the samples. In the ACRG cohort, GCs of the diffuse type are more represented. However, both identified the MSI subtype with hypermethylation of MHL1, high mutation frequency and a better prognosis. The EBV and MSS/TP53 + subtypes are similar in that many cases belonging to the MSS/TP53+ subtype is EBV+ and present mutations in PIK3CA and ARID1A. The GS and MSS/EMT subtypes, which include younger patients, are mostly diffuse and show low intercellular adhesion. The CIN and MSS/TP53- subtypes present with mutations in TP53 and amplification of members of the EGFR family, and are mostly intestinal.
Due to new technologies, such as NGS and microarray, recent discoveries have made possible to integrate diagnostic and therapeutic method, based on genotype and phenotype, and to apply them to individual patients with GC in the age of precision medicine.
Tumor markers are used to determine the clinical stage, assess the treatment response, and predict the risk of recurrence after treatment. Currently, markers such as α-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA-125), and carbohydrate antigen 19-9 (CA19-9) are frequently used in clinical practice. CEA is a risk factor for liver metastases[47], and increased CEA levels have been observed in all advanced GCs. The sensitivity and specificity of CEA for predicting GC recurrence is < 60% and < 80%, respectively[48]. CA19-9 is a marker commonly used in GC, although it is also present in other neoplastic pathologies. In combination with other tumor markers, CA19-9 can provide more information to predict GC recurrence[49]. Other markers such as AFP and CA-125 are widely used in the diagnosis of GC. AFP is an indicator of a high stage and presence of hepatic metastases[50], and CA-125 is associated with peritoneal diffusion[47].
Among the new biomarkers, human epidermal growth factor receptor 2 (encoded by ERBB2 commonly referred to as HER2) represents the first biomarker available in clinical practice for patients with GC. HER2 belongs to the EGFR family and has tyrosine kinase activity[51]. An estimated 6%-23% of GCs have overexpression and/or amplification of HER2[52,53], and it is mainly found in intestinal tumors[54,55]. HER2 is used in clinical practice for targeted therapy. EGFR or ERBB1 is expressed in about 30% of GCs[56]. The overexpression of EGFR in the pathogenesis of GC is associated with a poorly differentiated histology, vascular invasion, and shorter survival[57]. Tyrosine kinase inhibitors, particularly gefitinib and erlotinib, have shown efficacy in EGFR-amplified tumors. Mutations of EGFR confer resistance to these drugs[58,59]. In addition to anti-HER2 monoclonal antibodies, anti-EGFR therapy also includes gefitinib and erlotinib, tyrosine kinase inhibitors, as well as monoclonal antibodies such as cetuximab and panitumumab.
Other markers have attracted substantial attention as useful therapeutic candidates for targeted anti-cancer agents. For example, a high frequency (30%) of FGFR2 overexpression has been observed in GC. The amplification of FGFR2 is related to poor overall survival (OS)[60]. Furthermore, a study found that FGFR2 could be a biomarker for predicting the long-term failure of adjuvant chemotherapy for advanced GC[61]. Thus, FGFR2 may be a candidate for targeted anticancer agents. E-cadherin, a molecule involved in calcium-mediated cell adhesion, is a tumor suppressor whose deactivation is correlated with invasion and metastasis[62]. The deactivation of CDH1 may occur due to mutations, hypermethylation, loss of heterozygosity, and H. pylori infection[62]. Patients with CDH1 changes have generally worse survival than negative patients. It could be a useful marker for the diagnosis of preoperative biopsies[63]. Genetic deregulation of the PI3K/AKT/mTOR pathway has been frequently identified in GC[64,65], and mechanistic target of rapamycin kinase (mTOR) is activated in 60% of GCs[66].
Mutations of PI3KCA, which encodes the p110α catalytic isoform of PI3K, have been identified in up to 25% of patients with GC[67]. These mutations are involved in resistance to antitumor drugs and the acquisition of a metastatic phenotype; moreover, they are found mainly in the EBV-positive subtype of GC[68]. It has been reported that the amplification and/or overexpression of MET is involved in carcinogenesis, the efficacy of therapy, and the outcome of GC[69]. MET expression is associated with invasion and overall poor survival[70]. Vascular endothelial growth factor (VEGF) encodes for a growth factor that promotes the formation of new blood vessels. VEGF and vascular endothelial growth factor receptor (VEGFR) are upregulated in about 40% of GC cases[71], and their inhibition results in decreased cell proliferation and invasion. VEGF andsVEGFR2 are also used in clinical practice in targeted therapy[72,73]. TP53 is a tumor suppressor whose incidence of mutation in GC is about 3%-65%[74]. In the EBV-positive subtype, the incidence of TP53 mutation is lower[28]; moreover, an increased incidence has been observed in the intestinal type[75]. In many human tumors, TP53 mutations are associated with a poor prognosis[76]. For GC, there is no well-established clinical significance between the TP53 status and the outcome of patients. Recent studies, however, have integrated the mutational status of TP53 and other genetic alterations to define subpopulations of GCs in order to define the clinical relevance[77]. TP53 mutations appear to be a cofactor that supports the expression of genes involved in various signaling pathways; and whose aberrant activation leads to high proliferation, increased metastatic potential, and resistance to treatment. AURKA and MDM2 proto-oncogene (MDM2) encode negative regulators of TP53. AURKA is amplified and overexpressed in GC[78]. By regulating the ubiquitination of TP53 through MDM2, AURKA promotes tumor growth and cell survival[79].
DNA damage is repaired by a series of mechanisms, including basic excision repair, mismatch repair, nucleotide excision repair, single-strand annealing, homologous recombination, and non-homologous end joining. The poly (ADP-ribose) polymerases, known as PARPs, are proteins involved in the basic excision repair pathway and catalyze the transfer of ADP-ribose to target proteins[80,81]. PARP1 and PARP2 are the best known of these proteins. Numerous studies have highlighted an upregulation of PARPs in different tumors, including GC[82,83]. A high expression of PARP1 in GC is associated with tumor invasion and a poor prognosis[84].
Proteins in the matrix metalloproteinase (MMP) family are involved in breakdown of the extracellular matrix in normal physiologic processes and can promote cancer cell invasion and metastasis by degrading the extracellular matrix. Increased matrix metallopeptidase 15 (MMP15) expression is associated with poor prognosis in GC[85]. In addition, overexpression of matrix metallopeptidase 9 (MMP9) is a poor prognostic factor in patients with GC[86].
Fibrinogen C domain containing 1 (FIBCD1) is an acetyl group-binding receptor, which shows high affinity and calcium-dependent binding to acetylated structures such as chitin, some N-acetylated carbohydrates, and amino acids but not to their non-acetylated counterparts. The expression of FIBCD1 is significantly increased in GC tissues compared with normal tissues, and its overexpression is related to a poor prognosis[87]. FIBCD1 may be a novel prognostic marker in gastric GC; however, the mechanisms underlying its function require further studies.
PD-1 and PD-2 are the immune checkpoint receptors expressed on T and B lymphocytes, natural killer T cells, and monocytes[88]. After binding with PD-L1 and PD-L2 on activated T cells, they downregulate the activity of cytotoxic T cells and thus induce immunotolerance to the tumor. In 15%-70% of patients with GC, PD-L1 expression has been observed and this expression correlates with poor outcome[89]. Upregulation of PD-L1/PD-L2 expression in the EBV-positive subtype has been observed[29].
Circulating tumor cells (CTCs), single or in clusters, originate from primary tumor or metastases[90]. Clinically, they are related to the progression and metastatic processes, and therefore can be used as surveillance markers. CTCs can identify early stages of metastasis and thus identify patients who may benefit from treatment after primary tumor surgery[90,91]. The presence of CTCs, which have the characteristics of stem cell-like or EMT cells, allows evaluation of the tumor stage and the prediction of recurrence. Circulating cell-free DNA is more sensitive than CTCs, originates from normal and cancerous cells, and is present in the blood[92]. Circulating tumor DNA (ctDNA) originates from the primary tumor or metastases and can be used for the specificity of the diagnosis, even if the sensitivity is lower than the common markers used[93]. The ctDNA shows the presence of EBV DNA, and is useful for identifying EBV-positive subtypes[94]. The response to therapy can also be assessed with ctDNA.
MiRNAs are small, non-coding RNAs that, by regulating gene expression, play a role in the processes of proliferation, differentiation, and cell invasion[95]. They can increase the expression of oncogenes or reduce the expression of oncosuppressor genes[96]. Numerous miRNAs have been identified and play a role in GC[97,98]. Circulating cell-free miRNAs can be used as non-invasive biomarkers for the diagnosis and relapse of GC[99-101]. Approximately 135 long non-coding RNAs (lncRNAs), non-transcribed RNA sequences longer than 200 nucleotides, are dysregulated and strongly correlated with tumorigenesis, metastasis, and prognosis of GC[102-103]. Some lncRNAs are overexpressed in GC compared to healthy control tissue and may be prognostic markers[104,105]. However, further studies are needed to determine their possible clinical use.
Surgery is the elective treatment for many stages of GC. In a patient with GC at stage 0, I, II, or III, surgery (often together with other treatments) is currently the only treatment. Depending on the type and stage of GC, it is possible with surgery to remove all or part of the stomach including the nearby lymph nodes (the principles). Even when the tumor is too widespread to be removed entirely, patients can be helped by surgery because it can help prevent bleeding from the tumor or remove stomach obstruction due to tumor growth. This is termed palliative surgery because it allows the reduction or prevention of symptoms, but is not indicated for the treatment of GC[106].
Minimally invasive surgery, including laparoscopic gastrectomy and robotic gastrectomy, is receiving much attention in GC management[107,108]. The laparoscopic gastrectomy has the advantage of leading to a faster recovery with shorter hospital stays compared to the traditional surgery[109]. However, it has the disadvantage of limited movements. The robotic gastrectomy has overcome these limitations and its use is spreading rapidly[110-112]. Some studies have been carried out in order to compare both the advantages and disadvantages of two technologies[113-115]. The disadvantages of robotic gastrectomy concern its cost, duration of the procedure, and training needs[116]. Unfortunately, the lack of controlled and randomized studies has precluded the ability to establish a clear indication of robotic gastrectomy in the treatment of GC[116].
Surgical resection with pre- and post-operative chemotherapy and/or radiotherapy is the primary curative treatment of early-stage GC with a 5-year survival of about 30%[117-119]. Systemic chemotherapy is used to treat patients with localized and advanced GC. Palliative systemic therapy and chemo/radiotherapy are standard treatment options for patients with unresectable or metastatic advanced GC. Neoadjuvant chemotherapy with surgery is associated with the improved survival of patients with metastatic disease[120]. Perioperative chemotherapy with docetaxel, oxaliplatin, fluorouracil, and leucovorin (FLOT) significantly improves progression-free survival (referred to herein as PFS) and OS among patients with resectable GC compared with epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX)[121]. A Bayesian network meta-analysis obtained an estimate of the efficacy of perioperative FLOT and neoadjuvant treatments for resectable GC. Compared with surgery alone, perioperative cisplatin with fluorouracil (CF), perioperative ECF/ECX, and perioperative FLOT significantly improved survival. The most effective neoadjuvant treatment for the disease is likely to be perioperative FLOT[122]. Targeted therapy, a new therapeutic strategy, may improve the survival of patients with advanced GC. Clinical trials with targeted therapies have been performed in patients with GC. Table 1 shows some clinical trials, completed or ongoing, classified by specific molecular target.
| Signaling | Molecular target | Therapeutic agents | Clinical trial (Identifier) | Type of trial | Line of treatment | Phase | Patient's stage | Status | Ref. |
| EGFR | HER2 | Trastuzumab ± 5-FU/cisplatin/capecitabine | ToGA (NCT01041404) | Multicenter, randomized, open-label | First | III | Advanced gastric cancer, HER2-positive | Completed | [124] |
| HER2 | Trastuzumab/TS-1/cisplatin | NCT01736410 | Multicenter, non-randomized, open-label | First | II | Advanced gastric cancer, HER2-positive | Completed | [125] | |
| HER2 | Trastuzumab emtansine vs docetaxel-paclitaxel | GATSBY (NCT016441939) | Multicenter, randomized, open-label | Second | II/III | Advanced gastric cancer, HER2-positive | Completed | [126] | |
| HER2 | Pertuzumab ± trastuzumab/5-FU/cisplatin/capecitabine | JACOB (NCT01774786) | Multicenter, randomized, double | First | III | Metastatic gastric cancer, HER2-positive | Active | [127] | |
| HER2 | Lapatinib ± oxaliplatin/capecitabine | TRIO-013/LOGiC (NCT00680901) | Multicenter, randomized, quadruple | First | III | Advanced/metastatic gastric cancer, HER2-positive | Active | [128] | |
| HER2 | IMU-131 vaccine (HER-Vaxx) + 5-FU/cisplatin or capecitabine | NCT02795988 | Non-randomized, open-label | First/second | Ib/II | Advanced/metastatic gastric cancer, HER2-positive | Recruiting | [130] | |
| HER2 | Pyrotinib | (NCT02500199) | Open-label | Second | I | Advanced gastric cancer, HER2-positive | Recruiting | [131] | |
| HER2 | Pyrotinib ± docetaxel | (NCT02378389) | Open-label | Second | I | Advanced gastric cancer, HER2-positive | Recruiting | [132] | |
| EGFR | Cetuximab ± cisplatin/capecitabine | EXPAND (NCT00678535) | Multicenter, randomized, open-label | First | III | Advanced gastric cancer | Completed | [134] | |
| EGFR | Panitumumab + 5-FU/cisplatin/docetaxel | NCT01716546 | Multicenter, open-label | First | I/II | Locally advanced/metastatic gastric cancer | Terminated | [135] | |
| EGFR | Nimotuzumab + irinotecan vs irinotecan | ENRICH (NCT01813253) | Randomized, open-label | Second | III | Advanced/recurrent gastric cancer, EFGR overexpressed | Terminated | [136] | |
| EGFR | Nimotuzumab ± S-1/cisplatin | NCT02370849 | Randomized, open-label | First | II | Locally advanced/metastatic gastric cancer | Completed | [138] | |
| mTOR/PI3K/AKT | mTOR | Everolimus | NCT00519324 | Multicenter, open-label | Second/third | II | Advanced/metastatic, refractory gastric cancer | Completed | [142] |
| mTOR | Everolimus | NCT00729482 | Open-label | Second | II | Advanced, refractory gastric cancer | Completed | [143] | |
| mTOR | Everolimus + BSC vs placebo + BSC | GRANITE-1 (NCT00879333) | Multicenter, randomized, quadruple | Second/third | III | Advanced gastric cancer | Completed | [144] | |
| PI3KCA | Alpelisib + AUY922 | NCT01613950 | Multicenter, open-label | Second/third | Ib | Advanced/metastatic gastric cancer, PIK3CA mutations and/or HER2 amplification | Completed | [145] | |
| AKT | Ipatasertib ± 5FU/oxaliplatin/leucovorin | NCT01896531 | Randomized, double | Second | II | Advanced/metastatic gastric cancer | Active | [146] | |
| HGF/MET | HGF | Rilotumumab vs rilotumumab ± epirubicin/cisplatin/capecitabine | NCT00719550 | Multicenter, randomized | First | Ib/II | Locally advanced/metastatic gastric cancer | Completed | [149] |
| HGF | Rilotumumab ± epirubicin/cisplatin/capecitabine | RILOMET-1 (NCT01697072) | Multicenter, randomized, triple | First | III | Locally advanced/metastatic gastric cancer, MET-positive | Terminated | [150] | |
| HGF | Rilotumumab ± /cisplatin/capecitabine | RILOMET-2 (NCT02137343) | Multicenter, randomized, triple | First | III | Advanced gastric cancer | Terminated | [151] | |
| MET | Onartuzumab ± 5-FU/leucovorin/oxaliplatin | NCT01662869 | Multicenter, randomized, double | First | III | Metastatic gastric cancer, HER2 negative, MET-positive | Completed | [152] | |
| VEGF/VEGFR | VEGFR2 | Ramucirumab + BSC vs placebo + BSC | REGARD (NCT00917384) | Randomized, quadruple | First | III | Metastatic/locally recurrent gastric cancer | Completed | [72] |
| VEGFR2 | Ramucirumab ± paclitaxel | RAINBOW (NCT01170663) | Multicenter, randomized, double | Second | III | Metastatic, refractory gastric cancer | Completed | [73] | |
| VEGFR2 | Apatinib vs placebo | NCT01512745 | Randomized, quadruple | Third | III | Advanced/metastatic refractory gastric cancer | Completed | [156] | |
| TP53 | TP53 | Polymorphisms of xenobiotic metabolism, DNA repair, and TP53 genes | NCT01470404 | Gastric cancer treated with adjuvant chemotherapy | Completed | [161] | |||
| TP53 | APR-246 + 5-FU/cisplatin | NCT02999893 | Open-label | Second | I/II | Advanced/metastatic platinum resistant gastroesophageal cancer, TP53 mutated | Recruiting | [167] | |
| TP53 | AZD1775 (WEE inhibitor) + paclitaxel | NCT02448329 | Single center | Second | II | Advanced gastric cancer, TP53 mutated | Recruiting | [169] | |
| TP53 | HDM201 (inhibitor TP53/MDM2 interaction) | NCT02143635 | Multicenter, non-randomized, open-label | Second | I | Advanced/metastatic gastric cancer, TP53 wild-type | Active | [170] | |
| PARP | PARP | Olaparib + paclitaxel vs paclitaxel | Study 39 (NCT01063517) | Multicenter, randomized, double | Second | II | Metastatic/recurrent gastric cancer, low ATM expression | Active | [174] |
| PARP | Olaparib + paclitaxel vs placebo + paclitaxel | GOLD (NCT01924533) | Multicenter, randomized, double | Second | III | Advanced gastric cancer | Active | [175] | |
| PARP | Olaparib + ramucirumab | NCT03008278 | Open-label | Second | I/II | Metastatic/recurrent gastric cancer | Recruiting | [178] | |
| PARP | Veliparib + FOLFIRI | NCT01123876 | Open-label | First/second | I | Advanced gastric cancer | Completed | [179] | |
| Immune Checkpoint | PD-1 | Pembrolizumab vs pembrolizumab ± 5FU/cisplatin or capecitabine | KEYNOTE-059 (NCT02335411) | Multicenter, non-randomized, open-label | Second/third | II | Metastatic/recurrent gastric cancer | Active | [181] |
| PD-1 | Pembrolizumab vs paclitaxel | KEYNOTE-061 (NCT02370498) | Randomized, open-label | Second | III | Advanced gastric cancer | Active | [183] | |
| PD-1 | Pembrolizumab vs pembrolizumab ± 5FU/cisplatin or capecitabine | KEYNOTE-062 (NCT02494583) | Randomized, quadruple | First | III | Advanced gastric cancer | Active | [184] | |
| PD-1 | Pembrolizumab ± 5-FU/cisplatin or oxaliplatin/capecitabine | KEYNOTE-0859 (NCT03675737) | Randomized, double | First | III | Advanced/metastatic gastric cancer | Recruiting | [185] | |
| PD-1 | Pembrolizumab/trastuzumab ± cisplatin/capecitabine/oxaliplatin | NCT02954536 | Single group, open-label | First | II | Advanced/metastatic gastric cancer, HER2-positive | Recruiting | [186] | |
| PD-1 | Nivolumab vs placebo | ATTRACTION-2 (NCT02267343) | Multicenter, randomized, quadruple | Second | III | Advanced/recurrent gastric cancer | Active | [187] | |
| PD-1 | Nivolumab ± capecitabine/oxaliplatin | ATTRACTION-4 (NCT02746796) | Multicenter, randomized, quadruple | First | II/III | Advanced/recurrent refractory gastric cancer | Active | [188] | |
| PD-1 | Nivolumab ± ipilimumab | CHECKMATE-032 (NCT01928394) | Multicenter, randomized, open label | First/second | I/II | Advanced/metastatic, refractory gastric cancer | Active | [189] | |
| PD-1 | Nivolumab ± ipilimumab vs nivolumab + chemotherapy vs chemotherapy | CHECKMATE-649 (NCT02872116) | Multicenter, randomized, open-label | First | III | Advanced/metastatic gastric cancer | Recruiting | [190] | |
| PD-1 | Tislelizumab ± oxaliplatin/capecitabine or 5-FU/cisplatin | NCT03777657 | Randomized, triple | First | III | Locally advanced/metastatic gastric cancer | Recruiting | [191] |
The EGFR signaling pathway is activated in the GC[56,123]. Overexpression of EGFR has been associated with reduced OS[56,71]. This behavior may depend on the observation that EGFR targeting molecules may be potential agents for target therapy. Trastuzumab is the first molecular targeted agent approved as standard therapy for GC. It is a monoclonal antibody against HER2, which binds to the extracellular domain of the receptor. A phase III clinical trial (ToGA) (NCT01041404) enrolled 594 patients with GC who had high HER2 expression. These patients were randomized to chemotherapy alone or combined with trastuzumab. Treatment with trastuzumab led to an increase in OS of 2.7 mo and the PFS was heightened compared to that of patients treated with chemotherapy alone[124]. The benefits observed in patients treated with the combination of trastuzumab and chemotherapy were even more evident in patients who expressed high levels of HER2 compared to those with low HER2 expression. The 2015 National Comprehensive Cancer Network guidelines recommended the first-line treatment of trastuzumab combined with chemotherapy in patients overexpressing HER2. To date, trastuzumab is the only targeted therapy allowed for the treatment of advanced GC[124]. A clinical trial (NCT01736410), which evaluated the efficacy of trastuzumab with tegafur, gimeracil, oteracil (TS-1) and cisplatin as first-line treatment for advanced HER2-positive GC, has been completed. The combination of trastuzumab with TS-1 and cisplatin demonstrated good activity, was well tolerated, and is a first-line treatment that can be used for advanced HER2-positive GC[125]. In the GATSBY multicenter phase II/III study (NCT01641939), the efficacy of trastuzumab emtansine was evaluated in patients with advanced HER2-positive GC who had already received previous treatment. The results obtained were not encouraging since treatment with trastuzumab emtansine did not yield increases in OS compared to standard treatment with a taxane (docetaxel, paclitaxel)[126].
Other agents that target HER2, such as pertuzumab and lapatinib, have been used in clinical trials in patients with advanced GC and HER2 overexpression. One study (JACOB, NCT01774786) was performed with pertuzumab, trastuzumab, and chemotherapy in patients with untreated HER2-positive metastatic GC. This trial was the first to investigate the dual antibody blockade of HER2. Unfortunately, no significant improvement in OS was observed in the dual blockade group[127]. The clinical trial (TRIO-013/LOGiC, NCT00680901) performed with lapatinib in combination with oxaliplatin and capecitabine did not produce significant results in terms of OS[128]. A parallel study of biomarkers was conducted using immunohistochemistry and NGS. The most common alteration found in HER2-positive patients was amplification of CCNE1, which correlated with a lack of response to therapy. Patients with high levels of ERBB2 amplification were more responsive to therapy. The analysis of cell-free DNA showed that the amplification of ERBB2, detectable in the plasma of patients, was a predictive response. During disease progression, genetic changes were detected such as amplification of MYC, EGFR, FGFR2, and MET[129]. A phase I clinical trial (NCT02795988) evaluated the safety, tolerability, and immunogenicity of IMU-131, a peptide composed of three epitopes selected from the protein structure of HER2. In the phase II portion of the same trial, IMU-131 was used in combination with chemotherapy in patients overexpressing HER2. The study is ongoing, as only phase I has been completed, and no conclusions have been drawn[130]. Pyrotinib is an irreversible inhibitor of both HER2 and EGFR. The phase I studies (NCT02500199, NCT02378389) with pyrotinib and pyrotinib plus docetaxel in patients with HER2-positive GC are recruiting[131,132].
Studies have also been performed to identify markers to be used in monitoring the efficacy of trastuzumab alone or in combination with chemotherapy. Resistance has occurred in patients treated with trastuzumab. One of the main mechanisms that lead to this resistance are mutations in PI3KCA and PTEN[64,65,67]. The combination of trastuzumab with PI3K inhibitors may bring substantial benefits to patients with HER2-positive GC. One of the markers of resistance to trastuzumab is CCNE1, whose amplification is negatively correlated with the response to therapy directed against HER2[133]. Other monoclonal antibodies used to target EGFR include cetuximab and panitumumab. The results showed that anti-EGFR antibodies did not provide further benefits for patients with advanced GC receiving chemotherapy as first-line treatment (EXPAND) (NCT00678535)[134]. Panitumumab was used as first-line treatment in a clinical phase I/II trial (NCT01716546) in association with 5-FU, cisplatin, and docetaxel for locally advanced or metastatic GC. However, this study did not reach its primary endpoint because in an intermediate analysis, the number of responses obtained was lower than the prefixed limit[135]. Nimotuzumab is the first EGFR humanized monoclonal antibody that binds with high specificity to the extracellular region of EGFR. Two clinical trials have been concluded. The phase III study (NCT01813253) was performed to evaluate the OS in advanced GC patients with EGFR overexpression who were treated with nimotuzumab in combination with irinotecan and compared to a group of patients who received only irinotecan. This study, completed in 2018, has not yet had its results reported[136]. The second study (NCT02370849) evaluated the efficacy of cisplatin and S-1 with and without nimotuzumab in patients with advanced GC who were not previously treated[137]. The combination of nimotuzumab and S-1-cisplatin provided no additional benefit compared to chemotherapy alone in the first-line treatment of unresectable or metastatic GC[138]
Everolimus (RAD001) is an mTOR inhibitor with antitumor activity. In a phase I clinical trial, RAD001 was used in combination with capecitabine in patients with refractory GC; the clinical benefits were modest[139]. In phase I clinical trials (NCT01049620) and (NCT01042782), RAD001 was used in combination with capecitabine and oxaliplatin and with mitomycin C, respectively, in patients with advanced GC; the results of these trials are unknown[140,141]. In a multicenter phase II study (NCT00519324), RAD001 was used in patients with metastatic GC with previous chemotherapy failure; particularly, 10.1 mo was the median OS for which the monotherapy with everolimus, in patients in which the previous chemotherapy had failed, showed a satisfying disease control rate[142]. Another clinical trial (NCT00729482) evaluated the efficacy of RAD001 as a monotherapy in patients with advanced GC in whom standard first-line treatment had failed. In addition to the efficacy of RAD001, the expression of markers was evaluated in order to identify biomarkers of response to therapy. Tumors that did not have mTOR pathway activation did not benefit from treatment with RAD001[143]. The median OS was lower than that reported in the study conducted by Doi et al[142]. The results of this study showed that the efficacy of RAD001 was unsatisfactory compared to conventional treatment for advanced GC[143]. In the phase III GRANITE-1 study (NCT00879333), the median OS in patients treated with RAD001 vs placebo was 5.4 vs 4.3 mo. Compared to best supportive therapy (referred to as BSC in Table 1), RAD001 did not significantly improve OS in patients with advanced GC who were previously administered one or two lines of systemic chemotherapy[144]. A clinical trial (NCT01613950) was performed to investigate the efficacy of the combination of alpelisib (BYL719), a potent and selective inhibitor of mutated PI3KCA and AUY922, an inhibitor of heat shock protein 90 (HSP90), in patients with advanced GC with PIK3CA mutations and/or amplification of HER2, respectively. The results are not yet known[145]. Ipatasertib (GDC-0068), an inhibitor of serine/threonine kinase (AKT), has been used in combination with 5-FU, folinic acid, and oxaliplatin (mFOLFOX6) in advanced or metastatic GC in a multicenter placebo-controlled clinical trial (NCT01896531). The trial is ongoing[146].
High MET expression has been observed in intestinal GC rather than in the diffuse type and in advanced stage disease[147]. MET positivity is a prognostic factor for OS in GC[148]. Patients with GC and MET expression can benefit from anti-MET drugs. Rilotumumab is a hepatocyte growth factor (HGF) monoclonal antibody that blocks binding between HGF and its receptor MET. The efficacy of first-line rilotumumab in patients with GC in combination with ECX was demonstrated in a phase Ib/II clinical study (NCT00719550). The group of patients who received ECX plus rilotumumab showed a better prognosis than placebo[149]. The RILOMET-1 clinical trial (NCT01697072) evaluated the efficacy of rilotumumab in combination with epirubicin, cisplatin, and capecitabine. Regarding OS, the addition of rilotumumab to chemotherapy did not bring about benefits compared to chemotherapy alone in MET-positive patients[150], unlike the phase II study in which OS was 10.6 vs 5.7 mo in MET-positive patients who received rilotumumab compared to the placebo group[149]. The multicenter phase III clinical trial, RILOMET-2 (NCT02137343), in which patients with advanced GC were treated first-line with rilotumumab plus cisplatin and capecitabine, was closed for a review of the safety of the study[151]. The randomized, multicenter study (NCT01662869) evaluated the efficacy of onartuzumab (monoclonal anti-MET antibody) in combination with mFOLFOX6 in patients with metastatic HER2-negative and MET-positive GC. Onartuzumab did not yield satisfactory results in combination with FOLFOX[152].
Antibodies against VEGF and VEGFR have shown anti-tumor effects in combination with chemotherapy as first and second-line treatments for GC. Bevacizumab, a humanized monoclonal antibody against VEGF, inhibits the VEGF/VEGFR signaling pathway[153]. A phase II study (NCT00447330) was performed in patients with metastatic GC in combination with capecitabine and oxaliplatin; an OS of 7.2 and 10.8 mo was demonstrated in the two groups of patients treated with chemotherapy alone and with the combination with bevacizumab, respectively[154]. Ramucirumab is a humanized monoclonal antibody specific for VEGFR2. By blocking downstream VEGFR2 signaling, ramucirumab provides antitumor effects both as a single agent (REGARD trial, NCT00917384)[72] and in combination with paclitaxel (RAINBOW trial, NCT01170663)[73] in patients with metastatic refractory GC. The median OS was significantly longer in the group of patients treated with ramucirumab plus paclitaxel (9.6 mo) compared to those treated with paclitaxel plus placebo (7.4 mo). Thus, ramucirumab may be a new second-line treatment for patients with metastatic GC. The addition of ramucirumab to FOLFOX (leucovorin, 5-FU, oxaliplatin) did not improve OS in patients with advanced GC[155]. In a phase III study (NCT01512745), apatinib vs placebo was used in patients with advanced/metastatic GC who failed two lines of chemotherapy. The median OS was 6.5 vs 4.7 mo[156]. Other studies with apatinib have been started but have not yet been completed regarding the use of apatinib alone as first-line maintenance treatment in patients with advanced GC (NCT03255811)[157] and as maintenance treatment with capecitabine (NCT03598348) after first-line chemotherapy[158]. The clinical trial (NCT03104283) assessed the efficacy and safety of apatinib as monotherapy in elderly advanced GC patients, and determined the relationship between VEGFR2 expression and efficacy of apatinib treatment[159]. In a retrospective study, the efficacy of the association of apatinib with docetaxel vs apatinib as monotherapy as a second- or third-line treatment in advanced GC was evaluated. The median OS was 3.3 vs 6.0 mo in patients with apatinib monotherapy and those with apatinib and docetaxel combination, respectively. Patients with advanced GC benefited more with the combination of apatinib and docetaxel than with apatinib monotherapy[160].
A pharmacogenomic study (NCT01470404) was performed to evaluate the effects of germline polymorphisms in xenobiotic metabolism genes on the toxicity profile, and the role of germline polymorphisms of genes involved in DNA repair and the TP53 tumor suppressor to predict disease recurrence and survival in GC patients treated with adjuvant chemotherapy[161]. TP53 mutations represent a very attractive target for cancer therapy. One of the objectives being pursued is to identify molecules that can restore the function of wild-type TP53. Among these, APR-246 was identified[162], and has already been tested in mouse models of cancer[163-165] and in phase I/II clinical trials on hematological and prostate malignancies[166]. A phase I/II study (NCT02999893) was prepared for the treatment of gastroesophageal tumors with mutated TP53[167]. Because TP53 mutations are still somewhat difficult to address adequately, identifying TP53-dependent targets may provide new opportunities for alternative targeted therapies. For example, targeting WEE1 G2 checkpoint kinase (WEE1), a protein kinase that plays a role in the G2-M cell cycle checkpoint, prevents cells from entering mitosis in response to DNA damage[168]. AZD1775, an inhibitor of WEE1, was used in the clinical trial (NCT02448329) as second-line therapy in combination with paclitaxel in GC harboring TP53 mutations[169]. Overexpression of AURKA improves the stabilization of MDM2 and promotes the degradation of TP53, inhibiting its proapoptotic function in response to chemotherapy[79]. This result justifies the use of AURKA inhibitors in the treatment of GC. The TP53/MDM2 interaction inhibitor (HDM201) was used in the clinical trial (NCT02143635) in patients with advanced GC characterized by wild-type TP53; this study is ongoing[170]. No clinical trial has been performed on the use of MMP inhibitors in GC.
In response to DNA damage, sensors and effectors are activated that induce cell cycle arrest, damage repair, and eventually cell apoptosis. PARP inhibitors act by preventing breakage of the single DNA strand and induce tumor cell death[171]. In vitro, gastric carcinoma cell lines, particularly those in which the ATM serine/threonine kinase expression levels are low, were sensitive to the action of olaparib (PARP inhibitor)[172]. In a phase II study, the efficacy of olaparib (AZD-221) plus paclitaxel was evaluated vs paclitaxel in patients with recurrent or metastatic GC whose ATM expression levels were low or undetectable (Study 39; NCT01063517)[173]. The combination of olaparib plus paclitaxel significantly improved OS compared to placebo/paclitaxel, both in the general population and in the population with low ATM levels (13.1 vs 8.3 mo)[174]. A multicenter phase III trial has evaluated the efficacy of olaparib in combination with paclitaxel vs placebo plus paclitaxel in patients with advanced GC who are progressing after first-line treatment (GOLD, NCT01924533). The OS did not differ between treatment groups in the overall population (median OS 8.8 mo in the olaparib group vs 6.9 mo in the placebo group or the negative ATM population (12.0 mo vs 10.0 mo)[173].
The GOLD study did not achieve its primary objective to show a significant improvement in OS with olaparib in the overall or ATM-negative population of patients with advanced GC. However, the study provided data on efficacy and safety related to the use of olaparib in combination with a chemotherapy drug and the study itself is foundational for other studies in this type of patients[175]. The GOLD trial has been negative for its endpoints of improved OS, both in overall patient population and the ATM-negative population. The differences between the GOLD trial and Study 39 are the enriched population of ATM-negative patients in Study 39 (51% vs 19%) with respect to the GOLD study. PARP inhibitors are effective in tumors with a definite molecular signature, so it may not be realistic to expect efficacy from olaparib in an unselected marker population[176].
Furthermore, the time of exposure to olaparib was shorter in the GOLD trial than in Study 39[177]. A phase I/II pilot study was prepared to analyze the efficacy of olaparib in combination with ramucirumab in patients with metastatic, recurrent or unresectable GC (NCT03008278). The study is currently recruiting[178]. Another phase I clinical trial (NCT01123876) studied the combination of veliparib (PARP inhibitor) with FOLFIRI in patients with advanced solid tumors, including GC[179]. The antitumor activity of veliparib in combination with FOLFIRI and the acceptable safety profile lay the foundation for further studies.
Pembrolizumab is the first immune checkpoint inhibitor approved by the United States Food and Drug Administration (FDA) for the treatment of advanced or metastatic GC[180]. In a multicenter phase II trial (KEYNOTE-059, NCT02335411), the efficacy of pembrolizumab alone was demonstrated in patients with advanced GC who had previously been treated[181]. Treatment with pembrolizumab showed a higher overall response rate in PD-L1-positive patients than in PD-L1-negative patients. Furthermore, in MSI-high patients, the response was higher than that in non-MSI high patients. These results suggest that PD-L1 and MSI levels may be predictive biomarkers of pembrolizumab efficacy[182]. In the trial KEYNOTE-061 (NCT02370498), which was performed in patients with advanced PD-L1-positive GC, the efficacy of second-line treatment of pembrolizumab vs paclitaxel was compared. Pembrolizumab did not significantly improve OS compared to paclitaxel but had a better safety profile than paclitaxel[183]. Pembrolizumab was used as first-line monotherapy or in combination with cisplatin, 5-FU, or capecitabine in patients with advanced PD-L1-positive GC (KEYNOTE-062, NCT02494583); the results of this study are not yet known[184]. In the clinical trial KEYNOTE-859 (NCT03675737), the efficacy of pembrolizumab in association with chemotherapy with cisplatin and 5-FU or oxaliplatin and capecitabine, in advanced/metastatic HER2-negative GC expressing PD-L1, will be evaluated[185]. Another clinical trial (NCT02954536) is evaluating the first-line efficacy of the combination of pembrolizumab and trastuzumab in combination with chemotherapy in patients with HER2-positive metastatic GC. Preliminary results have been obtained on the safety and efficacy of the treatment. Resistance phenomena have occurred because mutations of TP53 (63%) and KRAS (16%) and loss of ERBB2 amplification in disease progression have been observed[186]. Nivolumab is a monoclonal antibody that targets PD-1 and has received FDA approval for neoplastic pathologies. In a clinical trial (NCT02267343) performed in patients with locally advanced or metastatic GC refractory to chemotherapy, nivolumab was effective with improvement of the median OS[187]. In another clinical trial (NCT02746796), the efficacy of nivolumab in combination with chemotherapy as first-line treatment was tested in patients with advanced or recurrent non-resectable GC[188]. In the clinical trial CheckMate-032 (NCT01928394), performed on solid tumors including GC, the efficacy of nivolumab in combination with ipilimumab, an anti-cytotoxic T-lymphocyte associated protein 4 antibody (CTLA4), was evaluated. Nivolumab with ipilimumab demonstrated encouraging long-term OS in patients with GC refractory to chemotherapy[189]. In the clinical trial CheckMate-649 (NCT02872116), the efficacy of nivolumab as first-line treatment in combination with ipilimumab vs nivolumab plus chemotherapy vs chemotherapy alone, is being evaluated in patients with advanced or metastatic GC[190]. The clinical trial (NCT03777657) is evaluating the efficacy of tislelizumab, a humanized anti-PD1, in combination with oxaliplatin and capecitabine or 5-FU and cisplatin[191].
Clinical trials have been performed to study the significance of CTCs in advanced/metastatic GC. Some trials (NCT03156777[192], NCT01625702)[193] have been designed to evaluate CTCs as markers of prognosis and response to chemotherapy. In a clinical trial (NCT01625702) in HER2-positive patients, an increased HER2 extracellular domain was a predictor of a better prognosis. The elevated levels of HER2 after therapy were correlated with a negative therapeutic response[194]. Other trials have been designed to evaluate CTCs and cell-free DNA as clinical prognosis markers (NCT01299688)[195] and response to HER2 (NCT02610218)[196] or VEGFR (NCT02048540)[197] targeting. Only one clinical trial (NCT01848015) was designed to establish the predictive value of CTCs in the recurrence of advanced GC after radical resection[198]. Some of these trials have been completed, but the results are not yet known. A study conducted in patients with HER2-positive metastatic GC revealed that the ctDNA of these patients provided useful information for monitoring the response to trastuzumab, for the purpose of developing therapeutic strategies for HER2-positive but trastuzumab-resistant patients[199]. A phase III clinical study (NCT01178944) was performed to determine if miR-215-5p levels could be predictive of the response to pralatrexate (a folate analog metabolic inhibitor) in association with oxaliplatin in patients with non-resectable GC[200]. Another study (NCT03253107) was conducted to determine if miRNAs levels may be predictive biological markers for the response to chemotherapy[201]. The results of these trials are not yet known. A study (NCT03057171) is ongoing on the control of H. pylori on the expression of lncRNAs in gastrointestinal diseases including GC[202].
GC is the fifth most malignant tumor worldwide and the third leading cause of cancer-related deaths[7]. Unfortunately, the disease becomes symptomatic in the advanced stage. GC is a complex disease whose onset is linked to a series of environmental and genetic factors[1-6]. Despite the increasing knowledge and progress in drug development, due to late diagnosis and extreme intra- and inter-tumor heterogeneity, the prognosis of GC patients is poor. The heterogeneity of GC is mainly linked to genetic and epigenetic alterations, but also interactions with the microenvironment and the presence of intratumoral cellular clones. Hence, there are variations between patients and within the same tumor. The new classifications, TCGA and ACRG, based on molecular profiles and complementary to those based on pathological characteristics[15], have highlighted four GC subtypes, each characterized by specific genetic alterations[29]. The molecular classification of GC has helped to identify molecular alterations that may be targeted by the therapy. Furthermore, the molecular profiles of GCs obtained from individual patients has provided new opportunities to identify biomarkers that may predict the tumor response to treatment[22-24]. Unfortunately, even today, the molecular characteristics of tumors are not taken into significant consideration in the management of patients.
H. pylori is responsible for the onset of peptic ulcers and 80% of GC cases. Eradication of the H. pylori infection treats gastritis and peptic ulcers and is a mean to prevent GC. Obviously, for the treatment of the eradication of the H. pylori, guidelines have been issued by three separate authoritative groups[203-205] but none overcome the problem of resistance. Fallone et al[206] recently revised the guidelines to arrive at the best treatment options; however, GC still develops after the eradication. Many Japanese investigators have reported that the presence of severe atrophy after eradication represents a risk factor for the development of GC[207]. Hence, there is a need for specific endoscopic surveillance programs for this type of patients.
Endoscopy plays an important role in the diagnosis of GC[208]. More than 90% of GC cases are reportedly revealed by biopsy-associated endoscopy. The increased use of endoscopy, thanks also to the revolutionary developments that have occurred recently and that have produced new, more sophisticated systems, has allowed highlighting of the “early” GC[209,210]. Ultrasonographic endoscopy is useful for TNM staging of GC patients, having a high diagnostic value. This technique allows the patient to be managed for the most appropriate treatment, limiting the occurrence of unnecessary exploratory surgical procedures[211]. Endoscopy can also be curative for early GC or used as palliative care for more advanced cases. In early GC, the endoscopic mucosal resection provides similar effects as traditional surgical resection[212,213].
Surgical resection with adjuvant or neoadjuvant radiotherapy and chemotherapy with cisplatin, 5-FU, taxane, or irinotecan, remains the most effective treatment for advanced GC. The recent MRC MAGIC/UK study (ISRCTN93793971) showed that perioperative ECF/ECX chemotherapy led to an improvement in OS and PFS in patients with resectable GC[214]. Perioperative chemotherapy is the standard of care in most of Europe for localized GC with accepted ECF or ECX regimens[215]. However, objective response rates to chemotherapy range from 20% to 40%, indicating variable clinical responses that are mostly likely caused by the biologic heterogeneity of the tumor. As with chemotherapy, therapeutic regimens based on targeted therapy have recently been introduced, which makes use of small molecules or monoclonal antibodies that can act on specific molecules capable of modifying molecular pathways involved in proliferation, differentiation, and cell invasion.
Based on phase III clinical trials in patients with advanced/metastatic GC, trastuzumab (anti-HER2) and ramucirumab (anti-VEGFR2) have been approved as first- and second-line therapies in these patients[72-74,124]. However, the survival of patients receiving these therapies is not very high, and with the exception of HER2, there are no markers that can be used to evaluate the response to therapy. Data from preclinical studies have shown a relationship between HER2 overexpression and activation of angiogenesis in breast cancer cells[216]. A retrospective study showed significant efficacy of the combination of a biological therapy with ramucirumab with a chemotherapeutic (paclitaxel) in patients in whom trastuzumab therapy had failed[217]. This study has demonstrated the crosstalk between HER2 signaling and angiogenesis in GC, which can explain tumor survival. Therefore, trastuzumab resistance could be overcome by inhibiting the angiogenic pathway. An analysis of subgroups extrapolated from the RAINBOW study showed that patients who had already been treated with trastuzumab benefited from treatment with ramucirumab in combination with paclitaxel[218]. New studies will be needed to evaluate the efficacy of sequential blockades of both pathways to improve the survival of patients with GC.
Other monoclonal antibodies, such as cetuximab and panitumumab (anti-EGFR), have also been tested in advanced/metastatic GC but the results on survival rates have not been encouraging[134-135]. Unacceptable results were obtained with RAD001, an mTOR inhibitor. The efficacy of RAD001 is unsatisfactory compared to conventional treatment for advanced GCs[139,143]. Anti-MET monoclonal antibodies, such as rilotumumab and onartuzumab, in combination with chemotherapy, did not bring benefits compared to chemotherapy alone[149-152]. A study was prepared to more effectively target MET with a mixture of two humanized monoclonal antibodies that target two non-overlapping MET epitopes. The results represent efficacy data demonstrated on preclinical models and are part of a clinical trial (NCT02648724)[219] carried out on patients with NSLC and MET amplification[220]. The advantage of antibody mixtures is their ability to orchestrate the internalization of the receptor and its degradation more effectively than a single monoclonal antibody, as previously shown for the EGFR family[221].
PARP inhibitors are very effective in the treatment of ovarian and breast tumors in which DNA repair systems are altered and BRCA1/2 mutations are present, which makes them more sensitive to these inhibitors[222]. New biomarkers are being explored, which go beyond BRCA1/2 mutations and DNA repair mechanism deficits to stratify sensitive patients, new combinations of PARP inhibitors, and/or combinations with checkpoint inhibitors to determine who will be eligible for this treatment for other solid tumors, including GC[223]. It has been hypothesized that the inhibition of PARP may trigger mechanisms based on the recognition of new tumor cell antigens by the immune system, making the PARP inhibitors potential partners for combination with immune checkpoint inhibitors.
Recently, patients with GC have also been studied from an immunotherapy viewpoint. Pembrolizumab and nivolumab received FDA approval for GC[180]. Anti-PD1 antibodies have been used in phase II and phase III clinical trials and appear to be promising, especially in patients overexpressing PD-L1. Further clinical trials are underway to evaluate the efficacy of these antibodies in association with chemotherapy. At the same time, other pathways such as the TP53 signaling pathway, are being studied to identify inhibitory molecules[162-166]. Strategic opportunities can also be provided by studying the potential of biomarkers such as CTCs, ctDNA, miRNAs, and lncRNAs to predict response to therapy and resistance phenomena.
There is no doubt that targeted therapies allow patients to live longer, whether they are administered alone or in combination with chemotherapy. Today the probability of observing patients who survive several years after the diagnosis of cancer is much higher, thanks to the targeted therapies. The targeted therapies must be provided to groups of patients who can benefit from them, screened on the molecular profiles to which the therapy is effective. Molecular profiling regarding the overexpression and/or mutation of the targets must be carried out on tissue biopsies, both in resectable and unresectable patients, to establish the correct targeted therapy to be used alone or associated with chemotherapy. It is necessary to continue to study the heterogeneity of GC. The fact that GC has genetic variations between different patients and/or in the same patient during its progression and/or during or after therapy (conventional or targeted) should drive investigations into the molecular characteristics present in tumor tissue, and the use of circulating biomarkers to predict and monitor disease progression and response to therapy. Furthermore, the association of several markers should be considered in order to appropriately classify the tumor and to establish therapeutic strategies that increase survival rates.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmed M, Limpakan S, Yuan Y, Vilaichone R S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Qi LL
| 1. | Nguyen LT, Uchida T, Murakami K, Fujioka T, Moriyama M. Helicobacter pylori virulence and the diversity of gastric cancer in Asia. J Med Microbiol. 2008;57:1445-1453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 2. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3126] [Cited by in F6Publishing: 3021] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 3. | de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42:219-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 262] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 4. | Cover TL. Helicobacter pylori diversity and gastric cancer risk. MBio. 2016;7:e01869-e01815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 5. | Amieva M, Peek RM. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology. 2016;150:64-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 535] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 6. | Correa P. Gastric cancer: overview. Gastroenterol Clin North Am. 2013;42:211-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 370] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 7. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53206] [Cited by in F6Publishing: 50885] [Article Influence: 8480.8] [Reference Citation Analysis (44)] |
| 8. | Asaka M, Mabe K. Strategies for eliminating death from gastric cancer in Japan. Proc Jpn Acad Ser B Phys Biol Sci. 2014;90:251-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13286] [Cited by in F6Publishing: 13416] [Article Influence: 706.1] [Reference Citation Analysis (1)] |
| 10. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 900] [Cited by in F6Publishing: 1163] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 11. | Kaneko S, Yoshimura T. Time trend analysis of gastric cancer incidence in Japan by histological types, 1975-1989. Br J Cancer. 2001;84:400-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 475] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 13. | Muñoz N, Franceschi S. Epidemiology of gastric cancer and perspectives for prevention. Salud Publica Mex. 1997;39:318-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of tumours of the digestive system. IARC: Lyon, France 2010; 44-58. [Cited in This Article: ] |
| 15. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4011] [Cited by in F6Publishing: 4110] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 16. | Qiu MZ, Cai MY, Zhang DS, Wang ZQ, Wang DS, Li YH, Xu RH. Clinicopathological characteristics and prognostic analysis of Lauren classification in gastric adenocarcinoma in China. J Transl Med. 2013;11:58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, Edge SB, Yang HK. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010;116:5592-5598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 18. | Laurén PA, Nevalainen TJ. Epidemiology of intestinal and diffuse types of gastric carcinoma. A time-trend study in Finland with comparison between studies from high- and low-risk areas. Cancer. 1993;71:2926-2933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 19. | Ribeiro MM, Sarmento JA, Sobrinho Simões MA, Bastos J. Prognostic significance of Lauren and Ming classifications and other pathologic parameters in gastric carcinoma. Cancer. 1981;47:780-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 20. | Amorosi A, Bianchi S, Buiatti E, Cipriani F, Palli D, Zampi G. Gastric cancer in a high-risk area in Italy. Histopathologic patterns according to Lauren's classification. Cancer. 1988;62:2191-2196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 21. | Carneiro F, Seixas M, Sobrinho-Simões M. New elements for an updated classification of the carcinomas of the stomach. Pathol Res Pract. 1995;191:571-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Miura JT, Xiu J, Thomas J, George B, Carron BR, Tsai S, Johnston FM, Turaga KK, Gamblin TC. Tumor profiling of gastric and esophageal carcinoma reveal different treatment options. Cancer Biol Ther. 2015;16:764-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, Zhang S, Lee M, Wu J, Lim KH, Lei Z, Goh G, Lim QY, Tan AL, Sin Poh DY, Riahi S, Bell S, Shi MM, Linnartz R, Zhu F, Yeoh KG, Toh HC, Yong WP, Cheong HC, Rha SY, Boussioutas A, Grabsch H, Rozen S, Tan P. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 499] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 24. | Ali SM, Sanford EM, Klempner SJ, Rubinson DA, Wang K, Palma NA, Chmielecki J, Yelensky R, Palmer GA, Morosini D, Lipson D, Catenacci DV, Braiteh F, Erlich R, Stephens PJ, Ross JS, Ou SH, Miller VA. Prospective comprehensive genomic profiling of advanced gastric carcinoma cases reveals frequent clinically relevant genomic alterations and new routes for targeted therapies. Oncologist. 2015;20:499-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Luo B, Wang Y, Wang XF, Liang H, Yan LP, Huang BH, Zhao P. Expression of Epstein-Barr virus genes in EBV-associated gastric carcinomas. World J Gastroenterol. 2005;11:629-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 108] [Cited by in F6Publishing: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2009;24:354-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Geddert H, Zur Hausen A, Gabbert HE, Sarbia M. EBV-infection in cardiac and non-cardiac gastric adenocarcinomas is associated with promoter methylation of p16, p14 and APC, but not hMLH1. Anal Cell Pathol (Amst). 2010;33:143-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 15] [Reference Citation Analysis (0)] |
| 28. | Lee J, van Hummelen P, Go C, Palescandolo E, Jang J, Park HY, Kang SY, Park JO, Kang WK, MacConaill L, Kim KM. High-throughput mutation profiling identifies frequent somatic mutations in advanced gastric adenocarcinoma. PLoS One. 2012;7:e38892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4230] [Cited by in F6Publishing: 4299] [Article Influence: 429.9] [Reference Citation Analysis (2)] |
| 30. | Muro K, Bang Y, Shankaran V, Geva R, Catenacci DV, Gupta S, Eder JP, Berger R, Gonzales EJ, Pulini J, Ray AB, Doller-Filhart M, Emancipator K, Pathiraja K, Shu X, Koshiji MR, Cheng J, Chung HC. A Phase 1B study of pembrolizumab (Pembro; Mk-3475) in patients (pts) with advanced gastric cancer. Ann Oncol. 2017;25:2014. [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, McRee AJ, Lin CC, Pathiraja K, Lunceford J, Emancipator K, Juco J, Koshiji M, Bang YJ. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 838] [Article Influence: 104.8] [Reference Citation Analysis (1)] |
| 32. | Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, Chan TL, Kan Z, Chan AS, Tsui WY, Lee SP, Ho SL, Chan AK, Cheng GH, Roberts PC, Rejto PA, Gibson NW, Pocalyko DJ, Mao M, Xu J, Leung SY. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43:1219-1223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 550] [Cited by in F6Publishing: 589] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 33. | Velho S, Oliveira C, Ferreira A, Ferreira AC, Suriano G, Schwartz S, Duval A, Carneiro F, Machado JC, Hamelin R, Seruca R. The prevalence of PIK3CA mutations in gastric and colon cancer. Eur J Cancer. 2005;41:1649-1654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | Ratti M, Lampis A, Hahne JC, Passalacqua R, Valeri N. Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches. Cell Mol Life Sci. 2018;75:4151-4162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 35. | Jo YS, Kim MS, Lee JH, Lee SH, An CH, Yoo NJ. Frequent frameshift mutations in 2 mononucleotide repeats of RNF43 gene and its regional heterogeneity in gastric and colorectal cancers. Hum Pathol. 2015;46:1640-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Tamura G. Alterations of tumor suppressor and tumor-related genes in the development and progression of gastric cancer. World J Gastroenterol. 2006;12:192-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 150] [Cited by in F6Publishing: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 37. | Huo X, Xiao X, Zhang S, Zhou D, Chen Z. Association of intron microsatellite instability and exon mutational profile of TP53 in human gastric cancers. Anticancer Res. 2017;37:4507-4514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Polom K, Das K, Marrelli D, Roviello G, Pascale V, Voglino C, Rho H, Tan P, Roviello F. KRAS mutation in gastric cancer and prognostication associated with microsatellite instability status. Pathol Oncol Res. 2019;25:333-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, Lee KW, Kim EH, Yim SY, Lee SH, Cheong JH, Jeong W, Cho JY, Kim J, Chae J, Lee J, Kang WK, Kim S, Noh SH, Ajani JA, Lee JS. Clinical significance of four molecular subtypes of gastric cancer Identified by The Cancer Genome Atlas project. Clin Cancer Res. 2017;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 298] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 40. | Sepulveda AR, Del Portillo AJ. Molecular basis of diseases of the gastrointestinal tract. In: Coleman WB, Tsongalis GJ. Molecular Pathology (Second Edition). Elsevier 2018; 387-415. [Cited in This Article: ] |
| 41. | Oliveira C, Pinheiro H, Figueiredo J, Seruca R, Carneiro F. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015;16:e60-e70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 246] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 42. | Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, Siu HC, Deng S, Chu KM, Law S, Chan KH, Chan AS, Tsui WY, Ho SL, Chan AK, Man JL, Foglizzo V, Ng MK, Chan AS, Ching YP, Cheng GH, Xie T, Fernandez J, Li VS, Clevers H, Rejto PA, Mao M, Leung SY. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 765] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 43. | Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3628] [Cited by in F6Publishing: 3504] [Article Influence: 166.9] [Reference Citation Analysis (0)] |
| 44. | Thumkeo D, Watanabe S, Narumiya S. Physiological roles of Rho and Rho effectors in mammals. Eur J Cell Biol. 2013;92:303-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 45. | Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1071] [Cited by in F6Publishing: 1332] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 46. | Ang YL, Yong WP, Tan P. Translating gastric cancer genomics into targeted therapies. Crit Rev Oncol Hematol. 2016;100:141-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 309] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 48. | Marrelli D, Pinto E, De Stefano A, Farnetani M, Garosi L, Roviello F. Clinical utility of CEA, CA 19-9, and CA 72-4 in the follow-up of patients with resectable gastric cancer. Am J Surg. 2001;181:16-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Song YX, Huang XZ, Gao P, Sun JX, Chen XW, Yang YC, Zhang C, Liu HP, Wang HC, Wang ZN. Clinicopathologic and prognostic value of serum carbohydrate antigen 19-9 in gastric cancer: a meta-analysis. Dis Markers. 2015;2015:549843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 50. | Kono K, Amemiya H, Sekikawa T, Iizuka H, Takahashi A, Fujii H, Matsumoto Y. Clinicopathologic features of gastric cancers producing alpha-fetoprotein. Dig Surg. 2002;19:359-65; discussion 365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 601] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 52. | Sheng WQ, Huang D, Ying JM, Lu N, Wu HM, Liu YH, Liu JP, Bu H, Zhou XY, Du X. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol. 2013;24:2360-2364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 83] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 53. | Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 737] [Cited by in F6Publishing: 811] [Article Influence: 50.7] [Reference Citation Analysis (2)] |
| 54. | Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, Jäger E, Altmannsberger HM, Robinson E, Tafe LJ, Tang LH, Shah MA, Al-Batran SE. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol. 2012;23:2656-2662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 55. | Bozzetti C, Negri FV, Lagrasta CA, Crafa P, Bassano C, Tamagnini I, Gardini G, Nizzoli R, Leonardi F, Gasparro D, Camisa R, Cavalli S, Silini EM, Ardizzoni A. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer. 2011;104:1372-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 56. | Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52:738-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 57. | Wang KL, Wu TT, Choi IS, Wang H, Resetkova E, Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Albarracin CT. Expression of epidermal growth factor receptor in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Cancer. 2007;109:658-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 58. | Liu S, Yang H, Ge X, Su L, Zhang A, Liang L. Drug resistance analysis of gefitinib-targeted therapy in non-small cell lung cancer. Oncol Lett. 2016;12:3941-3943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10-i19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 401] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 60. | Betts G, Valentine H, Pritchard S, Swindell R, Williams V, Morgan S, Griffiths EA, Welch I, West C, Womack C. FGFR2, HER2 and cMet in gastric adenocarcinoma: detection, prognostic significance and assessment of downstream pathway activation. Virchows Arch. 2014;464:145-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Hosoda K, Yamashita K, Ushiku H, Ema A, Moriya H, Mieno H, Washio M, Watanabe M. Prognostic relevance of FGFR2 expression in stage II/III gastric cancer with curative resection and S-1 chemotherapy. Oncol Lett. 2018;15:1853-1860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Chan AO. E-cadherin in gastric cancer. World J Gastroenterol. 2006;12:199-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 80] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Corso G, Carvalho J, Marrelli D, Vindigni C, Carvalho B, Seruca R, Roviello F, Oliveira C. Somatic mutations and deletions of the E-cadherin gene predict poor survival of patients with gastric cancer. J Clin Oncol. 2013;31:868-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 64. | Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 404] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 65. | Wen YG, Wang Q, Zhou CZ, Qiu GQ, Peng ZH, Tang HM. Mutation analysis of tumor suppressor gene PTEN in patients with gastric carcinomas and its impact on PI3K/AKT pathway. Oncol Rep. 2010;24:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Lang SA, Gaumann A, Koehl GE, Seidel U, Bataille F, Klein D, Ellis LM, Bolder U, Hofstaedter F, Schlitt HJ, Geissler EK, Stoeltzing O. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int J Cancer. 2007;120:1803-1810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 67. | Matsuoka T, Yashiro M. The role of PI3K/Akt/mTOR signaling in gastric carcinoma. Cancers (Basel). 2014;6:1441-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 68. | Iizasa H, Nanbo A, Nishikawa J, Jinushi M, Yoshiyama H. Epstein-Barr Virus (EBV)-associated gastric carcinoma. Viruses. 2012;4:3420-3439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 69. | Lee HE, Kim MA, Lee HS, Jung EJ, Yang HK, Lee BL, Bang YJ, Kim WH. MET in gastric carcinomas: comparison between protein expression and gene copy number and impact on clinical outcome. Br J Cancer. 2012;107:325-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 70. | Noguchi E, Saito N, Kobayashi M, Kameoka S. Clinical significance of hepatocyte growth factor/c-Met expression in the assessment of gastric cancer progression. Mol Med Rep. 2015;11:3423-3431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Lieto E, Ferraraccio F, Orditura M, Castellano P, Mura AL, Pinto M, Zamboli A, De Vita F, Galizia G. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol. 2008;15:69-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 72. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J; REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1541] [Cited by in F6Publishing: 1463] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 73. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A; RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1613] [Cited by in F6Publishing: 1583] [Article Influence: 158.3] [Reference Citation Analysis (0)] |
| 74. | Oki E, Zhao Y, Yoshida R, Egashira A, Ohgaki K, Morita M, Kakeji Y, Maehara Y. The difference in p53 mutations between cancers of the upper and lower gastrointestinal tract. Digestion. 2009;79:33-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Endoh Y, Sakata K, Tamura G, Ohmura K, Ajioka Y, Watanabe H, Motoyama T. Cellular phenotypes of differentiated-type adenocarcinomas and precancerous lesions of the stomach are dependent on the genetic pathways. J Pathol. 2000;191:257-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 76. | Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15:2-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1136] [Cited by in F6Publishing: 1330] [Article Influence: 120.9] [Reference Citation Analysis (0)] |
| 77. | Park S, Lee J, Kim YH, Park J, Shin JW, Nam S. Clinical relevance and molecular phenotypes in gastric cancer, of TP53 mutations and gene expressions, in combination with other gene mutations. Sci Rep. 2016;6:34822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Sakakura C, Hagiwara A, Yasuoka R, Fujita Y, Nakanishi M, Masuda K, Shimomura K, Nakamura Y, Inazawa J, Abe T, Yamagishi H. Tumour-amplified kinase BTAK is amplified and overexpressed in gastric cancers with possible involvement in aneuploid formation. Br J Cancer. 2001;84:824-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 160] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 79. | Sehdev V, Katsha A, Arras J, Peng D, Soutto M, Ecsedy J, Zaika A, Belkhiri A, El-Rifai W. HDM2 regulation by AURKA promotes cell survival in gastric cancer. Clin Cancer Res. 2014;20:76-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 80. | Amé JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1074] [Cited by in F6Publishing: 1123] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 81. | D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999;342:249-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 763] [Cited by in F6Publishing: 773] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 82. | Nomura F, Yaguchi M, Togawa A, Miyazaki M, Isobe K, Miyake M, Noda M, Nakai T. Enhancement of poly-adenosine diphosphate-ribosylation in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2000;15:529-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Ossovskaya V, Koo IC, Kaldjian EP, Alvares C, Sherman BM. Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in triple-negative breast cancer and other primary human tumor types. Genes Cancer. 2010;1:812-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 84. | Liu Y, Zhang Y, Zhao Y, Gao D, Xing J, Liu H. High PARP-1 expression is associated with tumor invasion and poor prognosis in gastric cancer. Oncol Lett. 2016;12:3825-3835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 85. | Xu X, Chen L, Xu B, Xie Q, Sun M, Deng X, Wu C, Jiang J. Increased MT2-MMP expression in gastric cancer patients is associated with poor prognosis. Int J Clin Exp Pathol. 2015;8:1985-1990. [PubMed] [Cited in This Article: ] |
| 86. | Chen J, Chen LJ, Zhou HC, Yang RB, Lu Y, Xia YL, Wu W, Hu LW. Prognostic value of matrix metalloproteinase-9 in gastric cancer: a meta-analysis. Hepatogastroenterology. 2014;61:518-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 87. | Jiang C, Zhu J, Zhou P, Zhu H, Wang W, Jin Q, Li P. Overexpression of FIBCD1 is predictive of poor prognosis in gastric cancer. Am J Clin Pathol. 2018;149:474-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1019] [Cited by in F6Publishing: 1126] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 89. | Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, Zhong X, Li X, Qian H, Wang X. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0182692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 176] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 90. | Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897-6904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1810] [Cited by in F6Publishing: 1867] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 91. | Glaves D. Correlation between circulating cancer cells and incidence of metastases. Br J Cancer. 1983;48:665-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 108] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 92. | Qi Q, Pan YF, Shen JJ, Gu XQ, Han SW, Liao HH, Jiang YZ, Zhong LP. Circulating DNA for detection of gastric cancer. Eur Rev Med Pharmacol Sci. 2016;20:2558-2564. [PubMed] [Cited in This Article: ] |
| 93. | Gao Y, Zhang K, Xi H, Cai A, Wu X, Cui J, Li J, Qiao Z, Wei B, Chen L. Diagnostic and prognostic value of circulating tumor DNA in gastric cancer: a meta-analysis. Oncotarget. 2017;8:6330-6340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 94. | Shoda K, Ichikawa D, Fujita Y, Masuda K, Hiramoto H, Hamada J, Arita T, Konishi H, Kosuga T, Komatsu S, Shiozaki A, Okamoto K, Imoto I, Otsuji E. Clinical utility of circulating cell-free Epstein-Barr virus DNA in patients with gastric cancer. Oncotarget. 2017;8:28796-28804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 95. | Han TS, Hur K, Xu G, Choi B, Okugawa Y, Toiyama Y, Oshima H, Oshima M, Lee HJ, Kim VN, Chang AN, Goel A, Yang HK. MicroRNA-29c mediates initiation of gastric carcinogenesis by directly targeting ITGB1. Gut. 2015;64:203-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 96. | Zhu X, Lv M, Wang H, Guan W. Identification of circulating microRNAs as novel potential biomarkers for gastric cancer detection: a systematic review and meta-analysis. Dig Dis Sci. 2014;59:911-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 97. | Wu HH, Lin WC, Tsai KW. Advances in molecular biomarkers for gastric cancer: miRNAs as emerging novel cancer markers. Expert Rev Mol Med. 2014;16:e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 98. | Liu HS, Xiao HS. MicroRNAs as potential biomarkers for gastric cancer. World J Gastroenterol. 2014;20:12007-12017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 75] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 99. | Sierzega M, Kaczor M, Kolodziejczyk P, Kulig J, Sanak M, Richter P. Evaluation of serum microRNA biomarkers for gastric cancer based on blood and tissue pools profiling: the importance of miR-21 and miR-331. Br J Cancer. 2017;117:266-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 100. | Huang Z, Zhu D, Wu L, He M, Zhou X, Zhang L, Zhang H, Wang W, Zhu J, Cheng W, Chen Y, Fan Y, Qi L, Yin Y, Zhu W, Shu Y, Liu P. Six serum-based miRNAs as potential diagnostic biomarkers for gastric cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:188-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 101. | Zhang Y, Guan DH, Bi RX, Xie J, Yang CH, Jiang YH. Prognostic value of microRNAs in gastric cancer: a meta-analysis. Oncotarget. 2017;8:55489-55510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 102. | Fang XY, Pan HF, Leng RX, Ye DQ. Long noncoding RNAs: novel insights into gastric cancer. Cancer Lett. 2015;356:357-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 103. | Song H, Sun W, Ye G, Ding X, Liu Z, Zhang S, Xia T, Xiao B, Xi Y, Guo J. Long non-coding RNA expression profile in human gastric cancer and its clinical significances. J Transl Med. 2013;11:225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 104. | Yuan CL, Li H, Zhu L, Liu Z, Zhou J, Shu Y. Aberrant expression of long noncoding RNA PVT1 and its diagnostic and prognostic significance in patients with gastric cancer. Neoplasma. 2016;63:442-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 105. | Fan QH, Yu R, Huang WX, Cui XX, Luo BH, Zhang LY. The has-miR-526b binding-site rs8506G > A polymorphism in the lincRNA-NR_024015 exon identified by GWASs predispose to non-cardia gastric cancer risk. PLoS One. 2014;9:e90008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 106. | Miner TJ, Karpeh MS. Gastrectomy for gastric cancer: defining critical elements of patient selection and outcome assessment. Surg Oncol Clin N Am. 2004;13:455-466, viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 107. | Koeda K, Nishizuka S, Wakabayashi G. Minimally invasive surgery for gastric cancer: the future standard of care. World J Surg. 2011;35:1469-1477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 108. | Parisi A, Nguyen NT, Reim D, Zhang S, Jiang ZW, Brower ST, Azagra JS, Facy O, Alimoglu O, Jackson PG, Tsujimoto H, Kurokawa Y, Zang L, Coburn NG, Yu PW, Zhang B, Qi F, Coratti A, Annecchiarico M, Novotny A, Goergen M, Lequeu JB, Eren T, Leblebici M, Al-Refaie W, Takiguchi S, Ma J, Zhao YL, Liu T, Desiderio J. Current status of minimally invasive surgery for gastric cancer: A literature review to highlight studies limits. Int J Surg. 2015;17:34-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 109. | Haverkamp L, Weijs TJ, van der Sluis PC, van der Tweel I, Ruurda JP, van Hillegersberg R. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc. 2013;27:1509-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 110. | Hashizume M, Sugimachi K. Robot-assisted gastric surgery. Surg Clin North Am. 2003;83:1429-1444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 111. | Woo Y, Hyung WJ, Pak KH, Inaba K, Obama K, Choi SH, Noh SH. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg. 2011;146:1086-1092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 112. | Park JY, Kim YW, Ryu KW, Eom BW, Yoon HM, Reim D. Emerging role of robot-assisted gastrectomy: analysis of consecutive 200 cases. J Gastric Cancer. 2013;13:255-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 113. | Kang BH, Xuan Y, Hur H, Ahn CW, Cho YK, Han SU. Comparison of surgical outcomes between robotic and laparoscopic gastrectomy for gastric cancer: the learning curve of robotic surgery. J Gastric Cancer. 2012;12:156-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 114. | Hyun MH, Lee CH, Kwon YJ, Cho SI, Jang YJ, Kim DH, Kim JH, Park SH, Mok YJ, Park SS. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol. 2013;20:1258-1265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 115. | Junfeng Z, Yan S, Bo T, Yingxue H, Dongzhu Z, Yongliang Z, Feng Q, Peiwu Y. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: comparison of surgical performance and short-term outcomes. Surg Endosc. 2014;28:1779-1787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 116. | Procopiuc L, Tudor Ş, Mănuc M, Diculescu M, Vasilescu C. Robot-assisted surgery for gastric cancer. World J Gastrointest Oncol. 2016;8:8-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (3)] |
| 117. | Rivera F, Vega-Villegas ME, López-Brea MF. Chemotherapy of advanced gastric cancer. Cancer Treat Rev. 2007;33:315-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 118. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11614] [Article Influence: 893.4] [Reference Citation Analysis (4)] |
| 119. | Wesolowski R, Lee C, Kim R. Is there a role for second-line chemotherapy in advanced gastric cancer? Lancet Oncol. 2009;903-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 120. | Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, Schmalenberg H, Luley KB, Prasnikar N, Egger M, Probst S, Messmann H, Moehler M, Fischbach W, Hartmann JT, Mayer F, Höffkes HG, Koenigsmann M, Arnold D, Kraus TW, Grimm K, Berkhoff S, Post S, Jäger E, Bechstein W, Ronellenfitsch U, Mönig S, Hofheinz RD. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: The AIO-FLOT3 trial. JAMA Oncol. 2017;3:1237-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 261] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 121. | Al-Batran SE, Homann N, Schmalemberg H, Kopp HG, Haag GM, Luley KB, Shmiegel WH, Folprecht G, Probst S, Prasnikar N, Tuss-Patience PC, Fischbach W, Trojan J, Koenigsmann M, Pauligk C, Goetze TO, Jaeger E, Meiler J, Schuler MH, Hofheinz RAl-Batran SE, Homa HR. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial. J Clin Oncol. 2017;35:4004. [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 122. | Cai Z, Yin Y, Zhao Z, Xin C, Cai Z, Yin Y, Shen C, Yin X, Wang J, Chen Z, Zhou Y, Zhang B. Comparative effectiveness of neoadjuvant treatments for resectable gastroesophageal cancer: a network meta-analysis. Front Pharmacol. 2018;9:872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Langer R, Von Rahden BH, Nahrig J, Von Weyhern C, Reiter R, Feith M, Stein HJ, Siewert JR, Höfler H, Sarbia M. Prognostic significance of expression patterns of c-erbB-2, p53, p16INK4A, p27KIP1, cyclin D1 and epidermal growth factor receptor in oesophageal adenocarcinoma: a tissue microarray study. J Clin Pathol. 2006;59:631-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 124. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4615] [Cited by in F6Publishing: 4839] [Article Influence: 345.6] [Reference Citation Analysis (1)] |
| 125. | Chua C, Tan IB, Yamada Y, Rha SY, Yong WP, Ong WS, Tham CK, Ng M, Tai DW, Iwasa S, Lim HY, Choo SP. Phase II study of trastuzumab in combination with S-1 and cisplatin in the first-line treatment of human epidermal growth factor receptor HER2-positive advanced gastric cancer. Cancer Chemother Pharmacol. 2015;76:397-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 126. | Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, Mansoor W, Chung HC, Bodoky G, Shitara K, Phillips GDL, van der Horst T, Harle-Yge ML, Althaus BL, Kang YK. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18:640-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 327] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 127. | Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, Song C, Wu H, Eng-Wong J, Kim K, Kang YK. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19:1372-1384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 292] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 128. | Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A, Salman P, Li J, Protsenko SA, Wainberg ZA, Buyse M, Afenjar K, Houé V, Garcia A, Kaneko T, Huang Y, Khan-Wasti S, Santillana S, Press MF, Slamon D. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial. J Clin Oncol. 2016;34:443-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 129. | Kim ST, Banks KC, Pectasides E, Kim SY, Kim K, Lanman RB, Talasaz A, An J, Choi MG, Lee JH, Sohn TS, Bae JM, Kim S, Park SH, Park JO, Park YS, Lim HY, Kim NKD, Park W, Lee H, Bass AJ, Kim K, Kang WK, Lee J. Impact of genomic alterations on lapatinib treatment outcome and cell-free genomic landscape during HER2 therapy in HER2+ gastric cancer patients. Ann Oncol. 2018;29:1037-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 130. | Wiedermann U, Good AJ, Garner-Spitzer E, Chao Y, Bulat I, Dechaphunkul A, Airpornwirat W, Charoentum C, Yen CJ, Yau TC, Maglakelidze M, Tanasanvimon S, Maneechavakajorn J, Sookprasert A, Bai LY, Chou WC, Ungtrakul T, Chong L, Ede N. A phase Ib/II open label study of IMU-131 HER2/Neu peptide vaccine plus cisplatin and either 5-fluorouracil or capecitabine chemotherapy in patients with HER2/Neu overexpressing metastatic or advanced adenocarcinoma of the stomach or gastroesophageal junc. J Clin Oncol. 2019;37 Suppl 4. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 131. | Wang J. Phase I study of pyrotinib in patients with HER2-positive solid tumors. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT02500199 ClinicalTrials.gov Identifier: NCT02500199. [Cited in This Article: ] |
| 132. | Yang Q. Study evaluating pyrotinib/pyrotinib in combination with docetaxel in patients with HER2+ advanced gastric cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT02378389 ClinicalTrials.gov Identifier: NCT02378389. [Cited in This Article: ] |
| 133. | Zhao H, Wang J, Zhang Y, Yuan M, Yang S, Li L, Yang H. Prognostic values of CCNE1 amplification and overexpression in cancer patients: a systematic review and meta-analysis. J Cancer. 2018;9:2397-2407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 134. | Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, Park JO, Sawaki A, Celik I, Götte H, Melezínková H, Moehler M; Arbeitsgemeinschaft Internistische Onkologie and EXPAND Investigators. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 589] [Cited by in F6Publishing: 640] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 135. | Kentepozidis N, Economopoulou P, Liontos M, Kotsakis A, Boukovinas I, Vardakis N, Kontopodis E, Prinarakis E, Skaltsi T, Souglakos J, Georgoulias V. Panitumumab in combination with modified docetaxel/cisplatin/5-fluorouracil as first-line treatment in gastric and gastroesophageal junction adenocarcinomas: a multicenter phase II study by the Hellenic Oncology Research Group. Ann Gastroenterol. 2018;31:698-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 136. | Kuhnil Pharmaceutical Co. L. Phase 3 study of nimotuzumab and irinotecan as second line with advanced or recurrect gastric and gastroesophageal junction cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT01813253 ClinicalTrials.gov Identifier: NCT01813253. [Cited in This Article: ] |
| 137. | Chi Y. Cisplatin and S-1 with or without nimotuzumab in untreated advanced gastric adenocarcinoma. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/show/NCT02370849 ClinicalTrials.gov Identifier: NCT02370849. [Cited in This Article: ] |
| 138. | Du F, Zheng Z, Shi S, Jiang Z, Qu T, Yuan X, Sun Y, Song Y, Yang L, Zhao J, Wang J, Chi Y. S-1 and cisplatin with or without nimotuzumab for patients with untreated unresectable or metastatic gastric cancer: a randomized, open-label phase 2 trial. Medicine (Baltimore). 2015;94:e958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 139. | Lim T, Lee J, Lee DJ, Lee HY, Han B, Baek KK, Ahn HK, Lee SJ, Park SH, Park JO, Park YS, Lim HY, Kim KM, Kang WK. Phase I trial of capecitabine plus everolimus (RAD001) in patients with previously treated metastatic gastric cancer. Cancer Chemother Pharmacol. 2011;68:255-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 140. | Kang YK. RAD001 in combination with capecitabine and oxaliplatin (XELOX) in patients with advanced gastric cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT01049620 ClinicalTrials.gov Identifier: NCT01049620. [Cited in This Article: ] |
| 141. | Al-Batran S. Phase I study of daily RAD001 in combination with mitomycin C in patients with advanced gastric cancer or cancer of the esophagogastric junction (S387). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT01042782 ClinicalTrials.gov Identifier: NCT01042782. [Cited in This Article: ] |
| 142. | Doi T, Muro K, Boku N, Yamada Y, Nishina T, Takiuchi H, Komatsu Y, Hamamoto Y, Ohno N, Fujita Y, Robson M, Ohtsu A. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol. 2010;28:1904-1910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 143. | Yoon DH, Ryu MH, Park YS, Lee HJ, Lee C, Ryoo BY, Lee JL, Chang HM, Kim TW, Kang YK. Phase II study of everolimus with biomarker exploration in patients with advanced gastric cancer refractory to chemotherapy including fluoropyrimidine and platinum. Br J Cancer. 2012;106:1039-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 144. | Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, Muro K, Kim YH, Ferry D, Tebbutt NC, Al-Batran SE, Smith H, Costantini C, Rizvi S, Lebwohl D, Van Cutsem E. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935-3943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 357] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 145. | Novartis Pharmaceuticals. PI3K inhibitor BYL719 in combination with the HSP90 inhibitor AUY922 in patients with advanced or metastatic gastric cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT01613950 ClinicalTrials.gov Identifier: NCT01613950. [Cited in This Article: ] |
| 146. | Genentech Inc. A study of GDC-0068 in combination with fluoropyrimidine plus oxaliplatin in participants with advanced or metastatic gastric or gastroesophageal junction cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/ NCT01896531 ClinicalTrials.gov Identifier: NCT01896531. [Cited in This Article: ] |
| 147. | Sotoudeh K, Hashemi F, Madjd Z, Sadeghipour A, Molanaei S, Kalantary E. The clinicopathologic association of c-MET overexpression in Iranian gastric carcinomas; an immunohistochemical study of tissue microarrays. Diagn Pathol. 2012;7:57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 148. | Fuse N, Kuboki Y, Kuwata T, Nishina T, Kadowaki S, Shinozaki E, Machida N, Yuki S, Ooki A, Kajiura S, Kimura T, Yamanaka T, Shitara K, Nagatsuma AK, Yoshino T, Ochiai A, Ohtsu A. Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastric Cancer. 2016;19:183-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 149. | Iveson T, Donehower RC, Davidenko I, Tjulandin S, Deptala A, Harrison M, Nirni S, Lakshmaiah K, Thomas A, Jiang Y, Zhu M, Tang R, Anderson A, Dubey S, Oliner KS, Loh E. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014;15:1007-1018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 150. | Catenacci DVT, Tebbutt NC, Davidenko I, Murad AM, Al-Batran SE, Ilson DH, Tjulandin S, Gotovkin E, Karaszewska B, Bondarenko I, Tejani MA, Udrea AA, Tehfe M, De Vita F, Turkington C, Tang R, Ang A, Zhang Y, Hoang T, Sidhu R, Cunningham D. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1467-1482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 243] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 151. | Amgen. A Phase 3 study of rilotumumab (AMG) with cisplatin and capecitabine (CX) as first-line therapy in gastric cancer (RILOMET-2). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT02137343 ClinicalTrials.gov Identifier: NCT02137343. [Cited in This Article: ] |
| 152. | Shah MA, Bang YJ, Lordick F, Alsina M, Chen M, Hack SP, Bruey JM, Smith D, McCaffery I, Shames DS, Phan S, Cunningham D. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol. 2017;3:620-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 218] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 153. | Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 154. | Uronis HE, Bendell JC, Altomare I, Blobe GC, Hsu SD, Morse MA, Pang H, Zafar SY, Conkling P, Favaro J, Arrowood CC, Cushman SM, Meadows KL, Brady JC, Nixon AB, Hurwitz HI. A phase II study of capecitabine, oxaliplatin, and bevacizumab in the treatment of metastatic esophagogastric adenocarcinomas. Oncologist. 2013;18:271-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 155. | Yoon HH, Bendell JC, Braiteh FS, Firdaus I, Philip PA, Cohn AL, Lewis N, Anderson DM, Arrowsmith E, Schwartz JD, Gao L, Hsu Y, Xu Y, Ferry D, Alberts SR, Wainberg ZA. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter Phase II trial. Ann Oncol. 2016;27:2196-2203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 156. | Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34:1448-1454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 551] [Cited by in F6Publishing: 652] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 157. | Dong M. Clinical study on treatment of apatinib mesylate in first-line maintenance of advanced gastric cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT03255811 ClinicalTrials.gov Identifier: NCT03255811. [Cited in This Article: ] |
| 158. | Yuan X. Evaluate the efficacy of maintenance treatment with capecitabine plus apatinib in advanced gastric cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT03598348 ClinicalTrials.gov Identifier: NCT03598348. [Cited in This Article: ] |
| 159. | Zhao J. Apatinib for the elderly advanced gastric cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT03104283 ClinicalTrials.gov Identifier: NCT03104283. [Cited in This Article: ] |
| 160. | Lin H, Han D, Fu G, Liu C, Wang L, Han S, Liu B, Yu J. Concurrent apatinib and docetaxel vs apatinib monotherapy as third- or subsequent-line therapy for advanced gastric adenocarcinoma: a retrospective study. Onco Targets Ther. 2019;12:1681-1689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 161. | Kang WK. Pharmacogenomic study (adjuvant chemotherapy). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT01470404 ClinicalTrials.gov Identifier: NCT01470404. [Cited in This Article: ] |
| 162. | Bykov VJ, Wiman KG. Mutant p53 reactivation by small molecules makes its way to the clinic. FEBS Lett. 2014;588:2622-2627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 163. | Zandi R, Selivanova G, Christensen CL, Gerds TA, Willumsen BM, Poulsen HS. PRIMA-1Met/APR-246 induces apoptosis and tumor growth delay in small cell lung cancer expressing mutant p53. Clin Cancer Res. 2011;17:2830-2841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 164. | Saha MN, Jiang H, Yang Y, Reece D, Chang H. PRIMA-1Met/APR-246 displays high antitumor activity in multiple myeloma by induction of p73 and Noxa. Mol Cancer Ther. 2013;12:2331-2341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 165. | Liang Y, Besch-Williford C, Hyder SM. PRIMA-1 inhibits growth of breast cancer cells by re-activating mutant p53 protein. Int J Oncol. 2009;35:1015-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 166. | Lehmann S, Bykov VJ, Ali D, Andrén O, Cherif H, Tidefelt U, Uggla B, Yachnin J, Juliusson G, Moshfegh A, Paul C, Wiman KG, Andersson PO. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol. 2012;30:3633-3639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 167. | Lipton L, King J. A study of APR-246 in oesophageal cancer (APROC). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT02999893 ClinicalTrials.gov Identifier: NCT02999893. [Cited in This Article: ] |
| 168. | Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1939] [Cited by in F6Publishing: 1954] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 169. | Ahn YJ, Lee J. Study of AZD1775 in combination with paclitaxel, in advanced gastric adenocarcinoma patients harboring TP53 mutation as a second-line chemotherapy. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT02448329 ClinicalTrials.gov Identifier: NCT02448329. [Cited in This Article: ] |
| 170. | Novartis Pharmaceuticals. Study to determine and evaluate a safe and tolerated dose of HDM201 in patients with selected advanced tumors that are TP53 wt. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT02143635 ClinicalTrials.gov Identifier: NCT02143635. [Cited in This Article: ] |
| 171. | Underhill C, Toulmonde M, Bonnefoi H. A review of PARP inhibitors: from bench to bedside. Ann Oncol. 2011;22:268-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 172. | Kubota E, Williamson CT, Ye R, Elegbede A, Peterson L, Lees-Miller SP, Bebb DG. Low ATM protein expression and depletion of p53 correlates with olaparib sensitivity in gastric cancer cell lines. Cell Cycle. 2014;13:2129-2137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 173. | Bang Y. Efficacy and safety study of olaparib in combination with paclitaxel to treat advanced gastric cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT01924533 ClinicalTrials.gov Identifier: NCT01924533. [Cited in This Article: ] |
| 174. | Bang YJ, Im SA, Lee KW, Cho JY, Song EK, Lee KH, Kim YH, Park JO, Chun HG, Zang DY, Fielding A, Rowbottom J, Hodgson D, O'Connor MJ, Yin X, Kim WH. Randomized, double-blind phase II trial with prospective classification by ATM protein level to evaluate the efficacy and tolerability of olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer. J Clin Oncol. 2015;33:3858-3865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 218] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 175. | Bang YJ, Xu RH, Chin K, Lee KW, Park SH, Rha SY, Shen L, Qin S, Xu N, Im SA, Locker G, Rowe P, Shi X, Hodgson D, Liu YZ, Boku N. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1637-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 203] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 176. | Smyth E. Missing a GOLDen opportunity in gastric cancer. Lancet Oncol. 2017;18:1561-1563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 177. | Bang YJ. Use of olaparib in patients with advanced gastric cancer - Authors' reply. Lancet Oncol. 2018;19:e76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 178. | Cecchini M. Olaparib and ramucirumab in treating patients with metastatic or locally recurrent gastric or gastroesophageal junction cancer that cannot be removed by surgery. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT03008278 ClinicalTrials.gov Identifier: NCT03008278. [Cited in This Article: ] |
| 179. | Berlin J, Ramanathan RK, Strickler JH, Subramaniam DS, Marshall J, Kang YK, Hetman R, Dudley MW, Zeng J, Nickner C, Xiong H, Komarnitsky P, Shepherd SP, Hurwitz H, Lenz HJ. A phase 1 dose-escalation study of veliparib with bimonthly FOLFIRI in patients with advanced solid tumours. Br J Cancer. 2018;118:938-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 180. | Fashoyin-Aje L, Donoghue M, Chen H, He K, Veeraraghavan J, Goldberg KB, Keegan P, McKee AE, Pazdur R. FDA approval summary: Pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist. 2019;24:103-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 181. | Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 883] [Cited by in F6Publishing: 1235] [Article Influence: 205.8] [Reference Citation Analysis (0)] |
| 182. | Ilson DH. Advances in the treatment of gastric cancer. Curr Opin Gastroenterol. 2018;34:465-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 183. | Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic C, Chung HC, Muro K, Goekkurt E, Mansoor W, McDermott RS, Shacham-Shmueli E, Chen X, Mayo C, Kang SP, Ohtsu A, Fuchs CS; KEYNOTE-061 investigators. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 721] [Cited by in F6Publishing: 870] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 184. | Merck Sharp Dohme Corp. Study of pembrolizumab (MK-3475) as first-line monotherapy and combination therapy for treatment of advanced gastric or gastroesophageal junction adenocarcinoma (MK-3475-062/KEYNOTE-062. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT02494583 ClinicalTrials.gov Identifier: NCT02494583. [Cited in This Article: ] |
| 185. | Merck Sharp Dohme Corp. Pembrolizumab (MK-3475) plus chemotherapy versus placebo plus chemotherapy in participants gastric or gastroesophageal junction (GEJ) adenocarcinoma (MK-3475-859/KEYNOTE-859). In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT03675737 ClinicalTrials.gov Identifier: NCT03675737. [Cited in This Article: ] |
| 186. | Janjigian YY, Chou JF, Simmons M, Momtaz P, Sanchez-Vega F, Shcherba M, Ku GY, Won E, Chong CR, Gerdes H, Kelsen DP, Ilson DH, Aljallad K, Segal MF, Millang BM, Schultz N, Shah PM, Solit DB, Capanu M, Frances J. First-line pembrolizumab (P), trastuzumab (T), capecitabine (C) and oxaliplatin (O) in HER2-positive metastatic esophagogastric adenocarcinoma (mEGA). J Clin Oncol. 2019;37:62-62. [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 187. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1283] [Cited by in F6Publishing: 1496] [Article Influence: 213.7] [Reference Citation Analysis (0)] |
| 188. | Chen LT, Kang YK, Tanimoto M BN. ATTRACTION-04 (ONO-4538-37): a randomized, multicenter, phase 2/3 study of nivolumab (Nivo) plus chemotherapy in patients (Pts) with previously untreated advanced or recurrent gastric (G) or gastroesophageal junction (GEJ) cancer. Ann Oncol. 2017;28:266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 189. | Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, de Braud F, Chau I, Harbison CT, Dorange C, Tschaika M, Le DT. CheckMate-032 study: Efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36:2836-2844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 473] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 190. | Janjian YY, Adenis A, Aucoin JS, Barone C, Boku N, Chau I, Cleary JM, Feeney K, Franke FA, Moehler M, Roca EL, Schenker M, Li M, Ajani JA. Checkmate 649: A randomized, multicenter, open-label, phase 3 study of nivolumab (Nivo) plus ipilimumab (Ipi) versus oxaliplatin plus fluoropyrimidine in patients (Pts) with previously untreated advanced or metastatic gastric (G) or gastroesophageal junct. J Clin Oncol. 2017;35. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 191. | Zhang C, Pandit N. Tislelizumab in combination with chemotherapy as first-line treatment in adults with inoperable, locally advanced or metastatic gastric, or gastroesophageal junction carcinoma. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT03777657 ClinicalTrials.gov Identifier: NCT03777657. [Cited in This Article: ] |
| 192. | Wei L. Application value of CTCs detection for advanced gastric cancer patients. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT03156777 ClinicalTrials.gov Identifier: NCT03156777. [Cited in This Article: ] |
| 193. | Shen L. Clinical significance of circulating tumor cells (CTCs) in blood of patients with advanced/metastatic gastric cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT01625702 ClinicalTrials.gov Identifier: NCT01625702. [Cited in This Article: ] |
| 194. | Li Y, Peng Z, Zhang X, Gong J, Shen L. Value of serum human epithelial growth factor receptor 2 extracellular domain and circulating tumor cells in evaluating therapeutic response in advanced gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:1293-1299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 195. | Kang WK. HER2 circulating tumor cells in gastric cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT01299688 ClinicalTrials.gov Identifier: NCT01299688. [Cited in This Article: ] |
| 196. | Shen L. Liquid biopsy in monitoring the therapeutic efficacy of targeted therapy in advanced/metastatic gastric cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT02610218 ClinicalTrials.gov Identifier: NCT02610218. [Cited in This Article: ] |
| 197. | Du N. Neoadjuvant Bev plus DOF vs DOF in LAGC and its association with circulating tumor cell. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT02048540 ClinicalTrials.gov Identifier: NCT02048540. [Cited in This Article: ] |
| 198. | Shen L. Circulating tumor cells (CTCs) in advanced gastric cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT01848015 ClinicalTrials.gov Identifier: NCT01848015. [Cited in This Article: ] |
| 199. | Shen L. Liquid biopsy: a powerful tool to monitor trastuzumab resistance in HER2-positive metastatic gastric cancer. Cancer Commun (Lond). 2018;38:72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 200. | Khushalani N. Pralatrexate and oxaliplatin in treating patients with unresectable or metastatic esophageal, stomach, or gastroesophageal junction cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT01178944 ClinicalTrials.gov Identifier: NCT01178944. [Cited in This Article: ] |
| 201. | Nam SY. Predicting biomarker of gastric cancer chemotherapy response. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT03253107 ClinicalTrials.gov Identifier: NCT03253107. [Cited in This Article: ] |
| 202. | Lee SK. A study on the gastrointestinal disease and Helicobacter Pylori controlled long non-coding RNA. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT03057171 ClinicalTrials.gov Identifier: NCT03057171. [Cited in This Article: ] |
| 203. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1710] [Cited by in F6Publishing: 1745] [Article Influence: 249.3] [Reference Citation Analysis (1)] |
| 204. | Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 744] [Cited by in F6Publishing: 847] [Article Influence: 121.0] [Reference Citation Analysis (1)] |
| 205. | Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, Jones NL, Render C, Leontiadis GI, Moayyedi P, Marshall JK. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151:51-69.e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 510] [Cited by in F6Publishing: 544] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 206. | Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology. 2019;157:44-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 207. | Shichijo S, Hirata Y. Characteristics and predictors of gastric cancer after Helicobacter pylori eradication. World J Gastroenterol. 2018;24:2163-2172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 43] [Cited by in F6Publishing: 36] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 208. | El Abiad R, Gerke H. Gastric cancer: endoscopic diagnosis and staging. Surg Oncol Clin N Am. 2012;21:1-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 209. | Song M, Ang TL. Early detection of early gastric cancer using image-enhanced endoscopy: Current trends. Gastrointest Interv. 2014;3:1-7. [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 210. | Akarsu M, Akarsu C. Evaluation of new technologies in gastrointestinal endoscopy. JSLS. 2018;22:00053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 211. | Tsendsuren T, Jun SM, Mian XH. Usefulness of endoscopic ultrasonography in preoperative TNM staging of gastric cancer. World J Gastroenterol. 2006;12:43-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 89] [Cited by in F6Publishing: 76] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 212. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1134] [Cited by in F6Publishing: 1104] [Article Influence: 48.0] [Reference Citation Analysis (4)] |
| 213. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 476] [Article Influence: 28.0] [Reference Citation Analysis (1)] |
| 214. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4120] [Cited by in F6Publishing: 4248] [Article Influence: 236.0] [Reference Citation Analysis (0)] |
| 215. | Mongan AM, Kalachand R, King S, O'Farrell NJ, Power D, Ravi N, Muldoon C, O'Byrne K, Reynolds JV. Outcomes in gastric and junctional cancer using neoadjuvant and adjuvant chemotherapy (epirubicin, oxaliplatin, and capecitabine) and radical surgery. Ir J Med Sci. 2015;184:417-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 216. | Alameddine RS, Otrock ZK, Awada A, Shamseddine A. Crosstalk between HER2 signaling and angiogenesis in breast cancer: molecular basis, clinical applications and challenges. Curr Opin Oncol. 2013;25:313-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 217. | Tehfe M, Tabchi S, Laterza MM, De Vita F. Ramucirumab in HER-2-positive gastroesophageal adenocarcinoma: an argument for overcoming trastuzumab resistance. Future Oncol. 2018;14:223-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 218. | De Vita F, Borg C, Farina G, Geva R, Carton I, Cuku H, Wei R, Muro K. Ramucirumab and paclitaxel in patients with gastric cancer and prior trastuzumab: subgroup analysis from RAINBOW study. Future Oncol. 2019;15:2723-2731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 219. | Patnaik A, Camidge D. Sym015 (Anti-MET) in patients with advanced solid tumor malignancies. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https//clinicaltrials.gov/show/NCT02648724 ClinicalTrials.gov Identifier: NCT02648724. [Cited in This Article: ] |
| 220. | Poulsen TT, Grandal MM, Skartved NJØ, Hald R, Alifrangis L, Koefoed K, Lindsted T, Fröhlich C, Pollmann SE, Eriksen KW, Dahlman A, Jacobsen HJ, Bouquin T, Pedersen MW, Horak ID, Lantto J, Kragh M. Sym015: a highly efficacious antibody mixture against MET-smplified tumors. Clin Cancer Res. 2017;23:5923-5935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 221. | Pedersen MW, Jacobsen HJ, Koefoed K, Dahlman A, Kjær I, Poulsen TT, Meijer PJ, Nielsen LS, Horak ID, Lantto J, Kragh M. Targeting three distinct HER2 domains with a recombinant antibody mixture overcomes trastuzumab resistance. Mol Cancer Ther. 2015;14:669-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 222. | Mateo J, Lord CJ, Serra V, Tutt A, Balmaña J, Castroviejo-Bermejo M, Cruz C, Oaknin A, Kaye SB, de Bono JS. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 413] [Cited by in F6Publishing: 393] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 223. | Pilié PG, Gay CM, Byers LA, O'Connor MJ, Yap TA. PARP inhibitors: extending benefit beyond BRCA-mutant cancers. Clin Cancer Res. 2019;25:3759-3771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |