Published online Oct 15, 2009. doi: 10.4251/wjgo.v1.i1.69
Revised: July 31, 2009
Accepted: August 7, 2009
Published online: October 15, 2009
AIM: To explore useful prognostic factors for mucinous adenocarcinoma (MAC) in the colon and rectum.
METHODS: MAC was divided into low- and high-grade types based on the degree of structural differentiation; low-grade MAC arisen from well to moderately differentiated adenocarcinoma and papillary carcinoma, and high-grade MAC from poorly differentiated adenocarcinoma and signet ring cell carcinoma. Immunohistochemically, the expression of 2 types of MUC1 (MUC1/DF and MUC1/CORE), MUC2, 2 types of MUC5AC (MUC5AC/CHL2 and HGM), MUC6, CDX2, and CD10 was examined in 16 cases of MAC consisting of 6 low- and 10 high-grade types.
RESULTS: MUC1/DF3 was expressed in 3 of 6 low-grade MAC (50%) and 10 of 10 high-grade MAC (100%). MUC1/CORE was expressed in 1 of 6 low-grade MAC (16.7%) and 7 of 10 high-grade MAC (70%). MUC2 was expressed in all MAC regardless of the grade. MUC5AC was expressed in 6 of 6 low-grade MAC (100%) and 4 of 10 high-grade MAC (40%). HGM was expressed in 5 of 6 low-grade MAC (83.3%) and 6 of 10 high-grade MAC (60%). Expression of MUC6 and CD10 was undetected in all MAC regardless of the grade. CDX2 was expressed in 5 of 6 low-grade MAC (83.3%) and 7 of 10 high-grade MAC (70%). Taken together, MUC1/DF3 was expressed significantly more frequently in high-grade MAC than in low-grade, and MUC5AC/CHL2 was expressed significantly more frequently in low-grade MAC than in high-grade.
CONCLUSION: It is proposed that MUC1/DF3 and MUC5AC/CHL2 immunostaining is useful to discriminate high-grade MAC from low-grade MAC.
- Citation: Onodera M, Nishigami T, Torii I, Sato A, Tao LH, Kataoka TR, Yoshikawa R, Tsujimura T. Comparison between colorectal low- and high-grade mucinous adenocarcinoma with MUC1 and MUC5AC. World J Gastrointest Oncol 2009; 1(1): 69-73
- URL: https://www.wjgnet.com/1948-5204/full/v1/i1/69.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v1.i1.69
Mucinous adenocarcinoma (MAC) is defined as a carcinoma with mucin composing more than 50% of the lesion and characterized by pools of extracellular mucin that contain malignant epithelium as acinar structures, strips of cells or single cells. MAC is not classified into subtypes in the World Health Organization Classification of Tumors of the Digestive System[1]. However, it has been reported that MAC can be divided into two types based on the degree of structural differentiation. One type of MAC is low-grade MAC arisen from well to moderately differentiated adenocarcinoma and papillary carcinoma, and the other high-grade MAC arisen from poorly differentiated adenocarcinoma and signet ring cell carcinoma[2].
Mucins are the major component in the mucus gel on epithelial surfaces with a characteristic organ- and cell type-specific distribution. MUC1 is a large cell surface mucin glycoprotein expressed by most glandular and ductal epithelial cells[3]. Normal stomach mucosa is characterized by the production of MUC5AC by the surface epithelial mucus cells and MUC6 by the gastric glands[4]. MUC2 is the secreted mucin present predominantly in small and large intestine and confined to goblet cells[4]. It has also been reported that altered mucin expression is a feature of precancerous and cancer cells. For example, the expression of MUC1 is up-regulated in a variety of carcinomas including colorectal carcinoma[5-8]. A decrease of MUC2 expression has been reported in poorly differentiated colorectal adenocarcinoma[8]. The gastric mucin MUC5AC has been reported to be expressed in colorectal adenocarcinoma[8]. In addition, CD10, a membrane-bound zinc metallopeptidase, is a small intestinal type-brush border marker and is expressed in the intestinal phenotype of gastric carcinoma[9]. The expression of CD10 has not been examined in colorectal MAC. CDX2, an intestine-specific transcription factor, is expressed in the nuclei of normal colorectal tissue, colorectal adenocarcinoma, and mucinous types of adenocarcinoma of ovarian and lung origin[10,11]. However, expression of these molecules has not been investigated to compare low-grade MAC with high-grade MAC. To find useful prognostic factors of colorectal MAC, we here immunohistochemically examine the expression of MUC1, MUC2, MUC5AC, CD10 and CDX2 in relation to the grade of colorectal MAC.
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. All patients provided written informed consent.
Sixteen patients with colorectal MAC underwent surgical resection at the Hospital of Hyogo College of Medicine between 2001 and 2005. These 16 MAC were divided into 6 low- and 10 high-grade tumors based on the degree of structural differentiation. Each specimen was examined by 2 authors (T.N. and T.T.) with a multiheaded microscope to reach a consensus on pathological diagnosis. Six patients were male and 10 female with a mean age of 62.1 ± 14.5 years (44-90 years) (Table 1).
| Case | Location | Sex | Age | Grade | MUC1/DF3 | MUC1/CORE | MUC2 | MUC5AC/CHL2 | HGM | MUC6 | CD10 | CDX2 |
| 1 | T | F | 71 | Low | - | - | ++ | + | + | - | - | - |
| 2 | R | M | 71 | Low | - | - | ++ | ++ | + | - | - | ++ |
| 3 | A | F | 83 | Low | + | - | ++ | ++ | ++ | - | - | ++ |
| 4 | A | F | 90 | Low | + | + | ++ | ++ | + | - | - | ++ |
| 5 | R | F | 48 | Low | + | - | ++ | + | ++ | - | - | ++ |
| 6 | R | M | 44 | Low | - | - | ++ | + | - | - | - | ++ |
| 7 | R | F | 75 | High | ++ | ++ | ++ | + | + | - | - | - |
| 8 | T | F | 57 | High | ++ | + | ++ | - | + | - | - | ++ |
| 9 | R | M | 47 | High | ++ | + | + | - | - | - | - | + |
| 10 | R | M | 63 | High | + | - | ++ | + | + | - | - | ++ |
| 11 | A | M | 57 | High | + | - | ++ | - | - | - | - | - |
| 12 | A | F | 68 | High | + | + | ++ | - | - | - | - | ++ |
| 13 | T | F | 48 | High | ++ | + | + | + | ++ | - | - | + |
| 14 | R | F | 75 | High | ++ | + | ++ | - | + | - | - | - |
| 15 | R | M | 48 | High | ++ | + | + | - | - | - | - | + |
| 16 | A | F | 48 | High | + | - | ++ | + | + | - | - | ++ |
The immunohistochemical analysis was carried out according to the method described previously[12]. Briefly, tissues embedded in paraffin were cut into 5-μm-thick sections. The sections were heated in 10 mmol/L sodium citrate buffer (pH 6.0) at 95°C for 40 min to facilitate antigen retrieval. For immunohistochemical analysis of MUC1, 2 monoclonal antibodies, MUC1/DF3 (15-3, Toray-fuji Bionics, Tokyo, Japan) that identified the core peptide of MUC1 with sialyl oligosaccharides and MUC1/CORE (Ma695, Novocastra Lab, Newcastle Upon Tyne, UK) that recognized the core peptide of MUC1, were used. For immunohistochemical analysis of MUC5AC, MUC5AC/CLH2 (CLH2, Novocastra Lab) that identified only MUC5AC without glycosidic modification and HGM (45M1, Novocastra Lab) that recognized MUC5AC regardless of glycosidic modification were used. The sections were incubated with the primary antibodies of MUC1/DF3, MUC1/CORE, MUC2 (Ccp58, Novocastra Lab), MUC5AC/CHL2, HGM, MUC6 (CLH5, Novocastra Lab), CD10 (56C6, Novocastra Lab), or CDX2 (CDX-2-88, Biogenex, SanRoman, CA, USA). Then, the sections were subjected to the Envision kit (Dako, Glostrup, Denmark) including the secondary antibodies. Immunoreacted cells were visualized with diaminobenzidine tetrahydrochloride, and nuclei were lightly counterstained with hematoxylin. Positive controls comprised normal pancreatic duct cells for MUC1/DF3 and MUC1/CORE, mature goblet cells of normal colonic mucosa for MUC2, normal gastric mucosa for MUC5AC/CLH2 and HGM, normal pyloric gland for MUC6, normal intestinal mucosa for CDX2, and normal small intestinal mucosa for CD10.
Immunohistochemical stains were graded by the presence of positively stained tumor cells as follow: -, less than 5% of tumor cells: +, 5% to 50% of tumor cells: and ++, over 50% of tumor cells. The cases showing + and ++ were evaluated as “positive”.
The value was shown as mean ± SE. Statistical analysis was carried out by the Student’s t-test or χ2 test (Excel: Microsoft, Redmond, WA, USA). A P-value below 0.05 was considered significant.
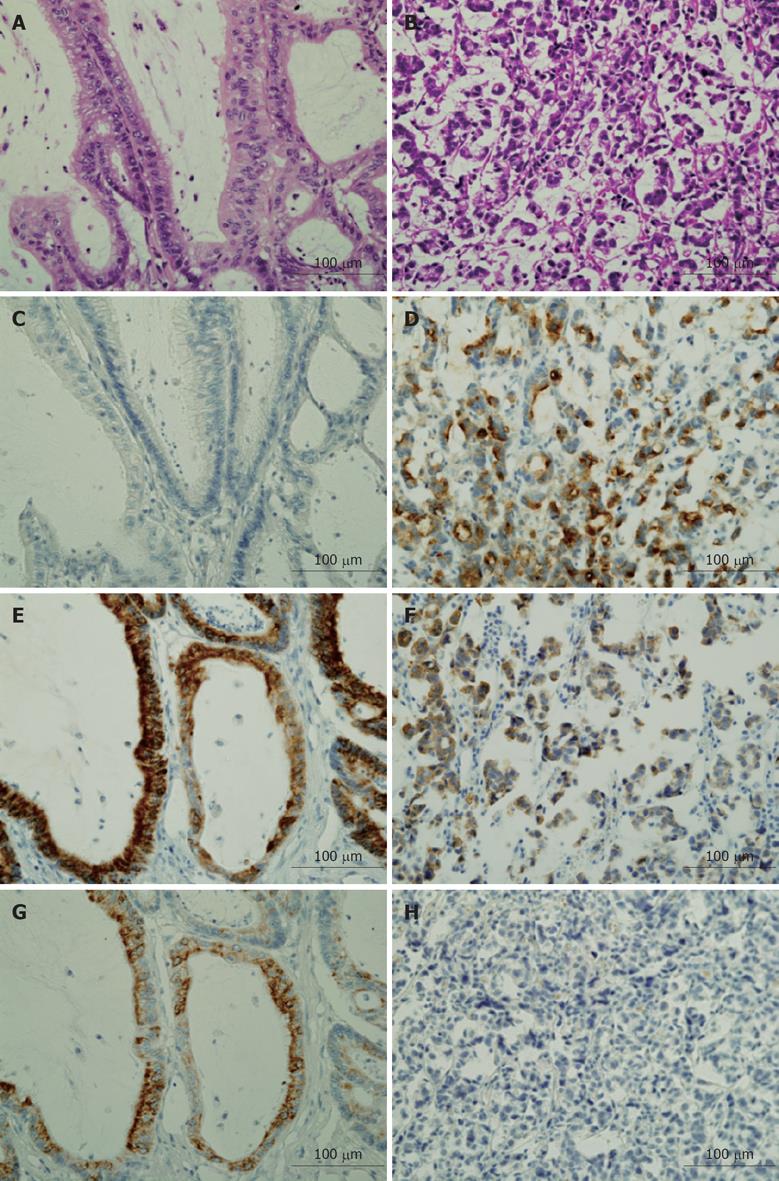
Clinical and immunohistochemical characteristics of each case are shown in Table 1. There was no significant difference between patients with low-grade MAC and those with high-grade MAC in both sex ratio (M:F = 2:4 vs 4:6) and age distribution (67.8 ± 7.53 vs 58.6 ± 3.53, P = 0.229 at t-test). MUC1/DF3 and MUC1/CORE were immunolocalized on the membrane and/or intracytoplasmic lumen of tumor cells. MUC1/DF3 was positive in 3 of 6 low-grade MAC (50%) and 10 of 10 high-grade MAC (100%). MUC1/CORE was positive in 1 of 6 low-grade MAC (16.7%) and 7 of 10 high-grade MAC (70%). MUC2 was immunolocalized in the cytoplasm of tumor cells. MUC2 was expressed in all MAC regardless of the grade. MUC5AC/CHL2 and HGM were immunolocalized in the cytoplasm of tumor cells with goblet or columnar cell features. MUC5AC/CHL2 was positive in 6 of 6 low-grade MAC (100%) and 4 of 10 high-grade MAC (40%). HGM was positive in 5 of 6 low-grade MAC (83.3%) and 6 of 10 high-grade MAC (60%). MUC6 and CD10 were not detected in any MAC regardless of the grade. CDX2 was immunolocalized in the nucleus of tumor cells. CDX2 was positive in 5 of 6 low-grade MAC (83.3%) and 7 of 10 high-grade MAC (70%). Taken together, MUC1/DF3 was expressed significantly more frequently in high-grade MAC than in low-grade (P = 0.131, χ2 test), and MUC5AC/CHL2 was expressed significantly more frequently in low-grade MAC than in high-grade (P = 0.164, χ2 test) (Table 2). Representative immunostaining patterns of MUC1/DF3, MUC2, and MUC5AC/CLH2 in low- and high-grade MAC are shown in Figure 1.
It has been reported that MUC1 is frequently expressed in invasive carcinomas, but not non-invasive carcinomas in various tissues, suggesting that the expression of MUC1 is related to increasing tendency for malignancy and invasion[13-15]. In the non-specific conventional adenocarcinoma of colon and rectum, MUC1 is considered to be a prognostic marker and served as a biological feature associated with the aggressiveness of advanced carcinomas[7]. We report that MUC1/DF3 was expressed significantly more frequently in high-grade MAC than in low-grade MAC in the colon and rectum. These results support that MUC1 is one of the indices of malignancy of tumors, and suggest that MUC1/DF3 immunostaining is useful to distinguish between low- and high-grade MAC.
MUC5AC is expressed in adenoma and conventional adenocarcinoma with well to moderate differentiation in the colon and rectum[16-20]. In addition, MUC5AC has also been reported to be expressed in 56%-63% cases of colorectal MAC[17,18]. On the other hand, Kocer et al[19] have reported that the absence of MUC5AC expression in tumors can be a prognostic factor for more aggressive adenocarcinoma in the colon and rectum. In this study, we found that the frequency of MUC5AC/CHL2 expression was significantly lower in high-grade MAC compared with low-grade MAC. These findings are consistent with the reports by Kocer et al[19], and indicate that decreases in MUC5AC expression are a prognostic marker for aggressive and advanced MAC.
MUC2 has been reported to be expressed in poorly differentiated adenocarcinoma in the colon and rectum[16-18]. In this study, MUC2 was expressed in all colorectal MAC regardless of the grade. These results indicate that MUC2 is a positive marker for colorectal MAC but is not suitable to distinguish between low-grade MAC and high-grade MAC. MUC6 and CD10 were not detected in any MAC regardless of the grade. These molecules may be negative markers for colorectal MAC.
In summary, we compared immunohistochemical expression of MUC1, MUC2, MUC5AC, MUC6, CD10 and CDX2 between low- and high-grade MAC, and found that increased MUC1 and decreased MUC5AC expressions are related to malignant potential of colorectal MAC. Since the expression of MUC1/DF3 and MUC5AC/CHL2 differed significantly between low- and high-grade MAC in the colon and rectum, it is proposed that MUC1/DF3 and MUC5AC/CHL2 immunostaining is useful to distinguish between these two types of MAC.
Mucinous adenocarcinoma (MAC) is characterized by pools of extracellular mucin that contain malignant epithelium. MAC can be divided into two types based on the degree of structural differentiation; low-grade MAC arisen from well to moderately differentiated adenocarcinoma and papillary carcinoma, and high-grade MAC arisen from poorly differentiated adenocarcinoma and signet ring cell carcinoma. However, useful markers for the differential diagnosis of low- and high-grade MAC in the colon and rectum have not been identified.
The immunohistochemical expression of 2 types of MUC1 (MUC1/DF and MUC1/CORE), MUC2, 2 types of MUC5AC (MUC5AC/CHL2 and HGM), MUC6, CDX2, and CD10 was compared between low- and high-grade MAC. MUC1/DF3 was expressed significantly more frequently in high-grade MAC than in low-grade, and MUC5AC/CHL2 was expressed significantly more frequently in low-grade MAC than in high-grade. These results indicate that increased MUC1 and decrease MUC5AC expressions are related to malignant potential of colorectal MAC.
It is proposed that MUC1/DF3 and MUC5AC/CHL2 immunostaining is useful to discriminate high-grade MAC from low-grade MAC in the colon and rectum.
MAC is defined as a carcinoma with mucin composing more than 50% of the lesion. Mucin, a high molecular weight glycoprotein, is the major component in the mucus gel on epithelial surfaces with a characteristic organ- and cell type-specific distribution. Mucin binds to pathogens as part of the immune system.
This is a good descriptive study in which authors compared the expression of mucins (MUC1, MUC2, MUC5AC, and MUC6), CD10, and CDX2 between low- and high-grade MAC. The results are interesting and indicate that MUC1 and MUC5AC are useful markers to discriminate high-grade MAC from low-grade MAC in the colon and rectum. The combination of markers in this study is quite unique.
Peer reviewer: Peter JK Kuppen, PhD, Associate Professor, Department of Surgery, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, The Netherlands
S- Editor Li LF L- Editor Lalor PF E- Editor Lin YP
| 1. | Hamilton SR, Aalton LA. Pathology and genetics of tumors of the digestive system. World Health Organization Classification of Tumors. Lyon: IARC Press 2000; 109. |
| 2. | Japanese society for Cancer of the Colon and Rectum. General rules for clinical and pathological studies on cancer of the colon, rectum and anus, 7th ed. Tokyo: Kaneharashuppan 2006; . |
| 3. | Gum JR Jr. Mucin genes and the proteins they encode: structure, diversity, and regulation. Am J Respir Cell Mol Biol. 1992;7:557-564. |
| 4. | Tsukashita S, Kushima R, Bamba M, Sugihara H, Hattori T. MUC gene expression and histogenesis of adenocarcinoma of the stomach. Int J Cancer. 2001;94:166-170. |
| 5. | Li A, Goto M, Horinouchi M, Tanaka S, Imai K, Kim YS, Sato E, Yonezawa S. Expression of MUC1 and MUC2 mucins and relationship with cell proliferative activity in human colorectal neoplasia. Pathol Int. 2001;51:853-860. |
| 6. | Zotter S, Lossnitzer A, Hageman PC, Delemarre JF, Hilkens J, Hilgers J. Immunohistochemical localization of the epithelial marker MAM-6 in invasive malignancies and highly dysplastic adenomas of the large intestine. Lab Invest. 1987;57:193-199. |
| 7. | Suzuki H, Shoda J, Kawamoto T, Shinozaki E, Miyahara N, Hotta S, Iizuka Y, Nakahara A, Tanaka N, Yanaka A. Expression of MUC1 recognized by monoclonal antibody MY.1E12 is a useful biomarker for tumor aggressiveness of advanced colon carcinoma. Clin Exp Metastasis. 2004;21:321-329. |
| 8. | Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77-99. |
| 9. | Wakatsuki K, Yamada Y, Narikiyo M, Ueno M, Takayama T, Tamaki H, Miki K, Matsumoto S, Enomoto K, Yokotani T. Clinicopathological and prognostic significance of mucin phenotype in gastric cancer. J Surg Oncol. 2008;98:124-129. |
| 10. | Drummond F, Putt W, Fox M, Edwards YH. Cloning and chromosome assignment of the human CDX2 gene. Ann Hum Genet. 1997;61:393-400. |
| 11. | Li MK, Folpe AL. CDX-2, a new marker for adenocarcinoma of gastrointestinal origin. Adv Anat Pathol. 2004;11:101-105. |
| 12. | Nishigami T, Onodera M, Torii I, Sato A, Tao LH, Kushima R, Kakuno A, Kishimoto M, Katsuyama E, Fujimori T. Comparison between mucinous cystic neoplasm and intraductal papillary mucinous neoplasm of the branch duct type of the pancreas with respect to expression of CD10 and cytokeratin 20. Pancreas. 2009;38:558-564. |
| 13. | Yonezawa S, Sato E. Expression of mucin antigens in human cancers and its relationship with malignancy potential. Pathol Int. 1997;47:813-830. |
| 14. | Yonezawa S, Taira M, Osako M, Kubo M, Tanaka S, Sakoda K, Takao S, Aiko T, Yamamoto M, Irimura T. MUC-1 mucin expression in invasive areas of intraductal papillary mucinous tumors of the pancreas. Pathol Int. 1998;48:319-322. |
| 15. | Higashi M, Yonezawa S, Ho JJ, Tanaka S, Irimura T, Kim YS, Sato E. Expression of MUC1 and MUC2 mucin antigens in intrahepatic bile duct tumors: its relationship with a new morphological classification of cholangiocarcinoma. Hepatology. 1999;30:1347-1355. |
| 16. | Park SY, Lee HS, Choe G, Chung JH, Kim WH. Clinicopathological characteristics, microsatellite instability, and expression of mucin core proteins and p53 in colorectal mucinous adenocarcinomas in relation to location. Virchows Arch. 2006;449:40-47. |
| 17. | Biemer-Hüttmann AE, Walsh MD, McGuckin MA, Simms LA, Young J, Leggett BA, Jass JR. Mucin core protein expression in colorectal cancers with high levels of microsatellite instability indicates a novel pathway of morphogenesis. Clin Cancer Res. 2000;6:1909-1916. |
| 18. | Ishizu H, Kumagai J, Eishi Y, Takizawa T, Koike M. Mucin core protein expression by colorectal mucinous carcinomas with or without mucus hyperplasia. J Gastroenterol. 2004;39:125-132. |
| 19. | Kocer B, Soran A, Erdogan S, Karabeyoglu M, Yildirim O, Eroglu A, Bozkurt B, Cengiz O. Expression of MUC5AC in colorectal carcinoma and relationship with prognosis. Pathol Int. 2002;52:470-477. |
| 20. | Nguyen MD, Plasil B, Wen P, Frankel WL. Mucin profiles in signet-ring cell carcinoma. Arch Pathol Lab Med. 2006;130:799-804. |