Copyright
©The Author(s) 2025.
World J Gastrointest Oncol. Aug 15, 2025; 17(8): 109646
Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.109646
Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.109646
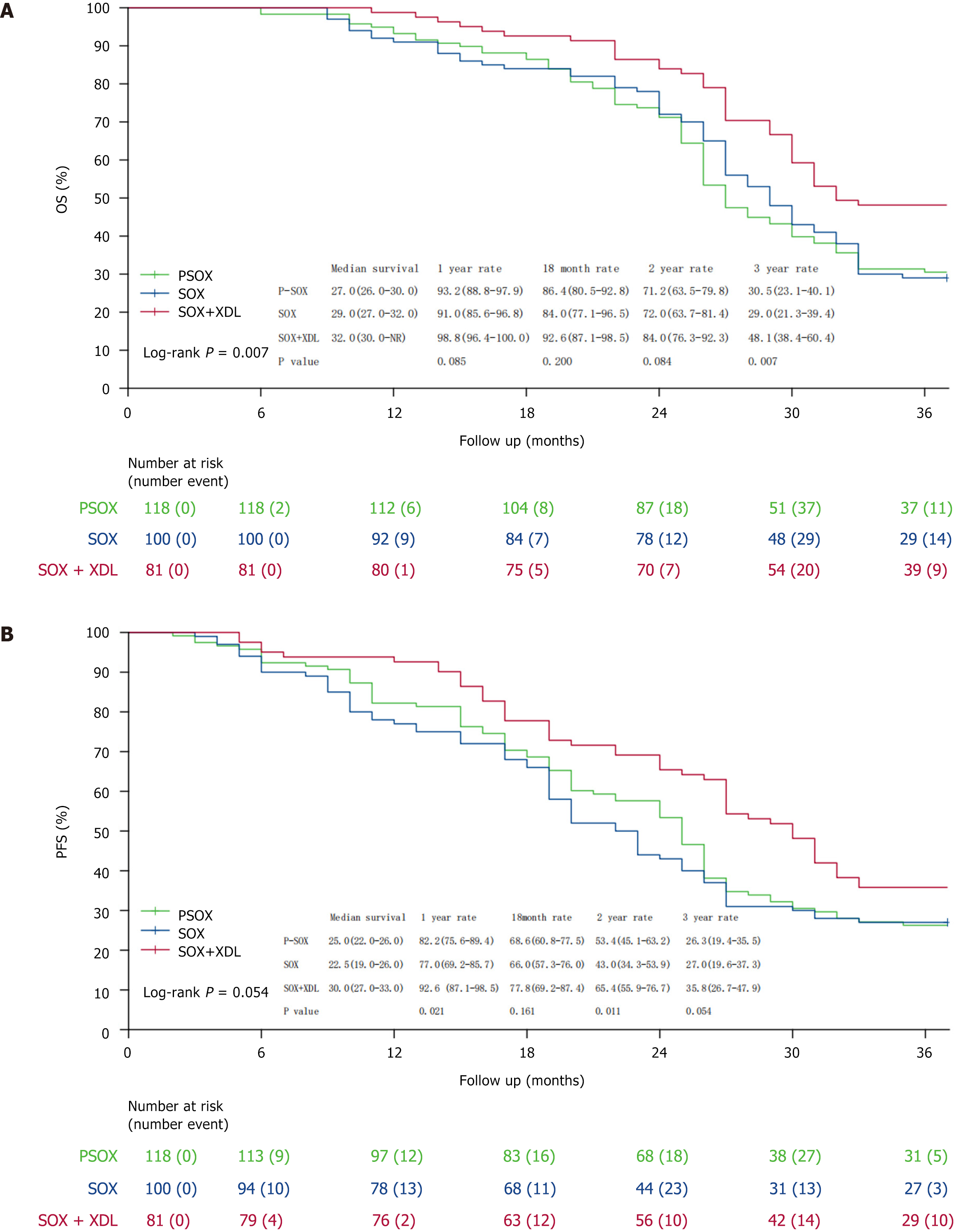
Figure 1 Comparison of overall survival and progression-free survival among the three groups.
A: Overall survival; B: Progression-free survival. OS: Overall survival; PFS: Progression-free survival.
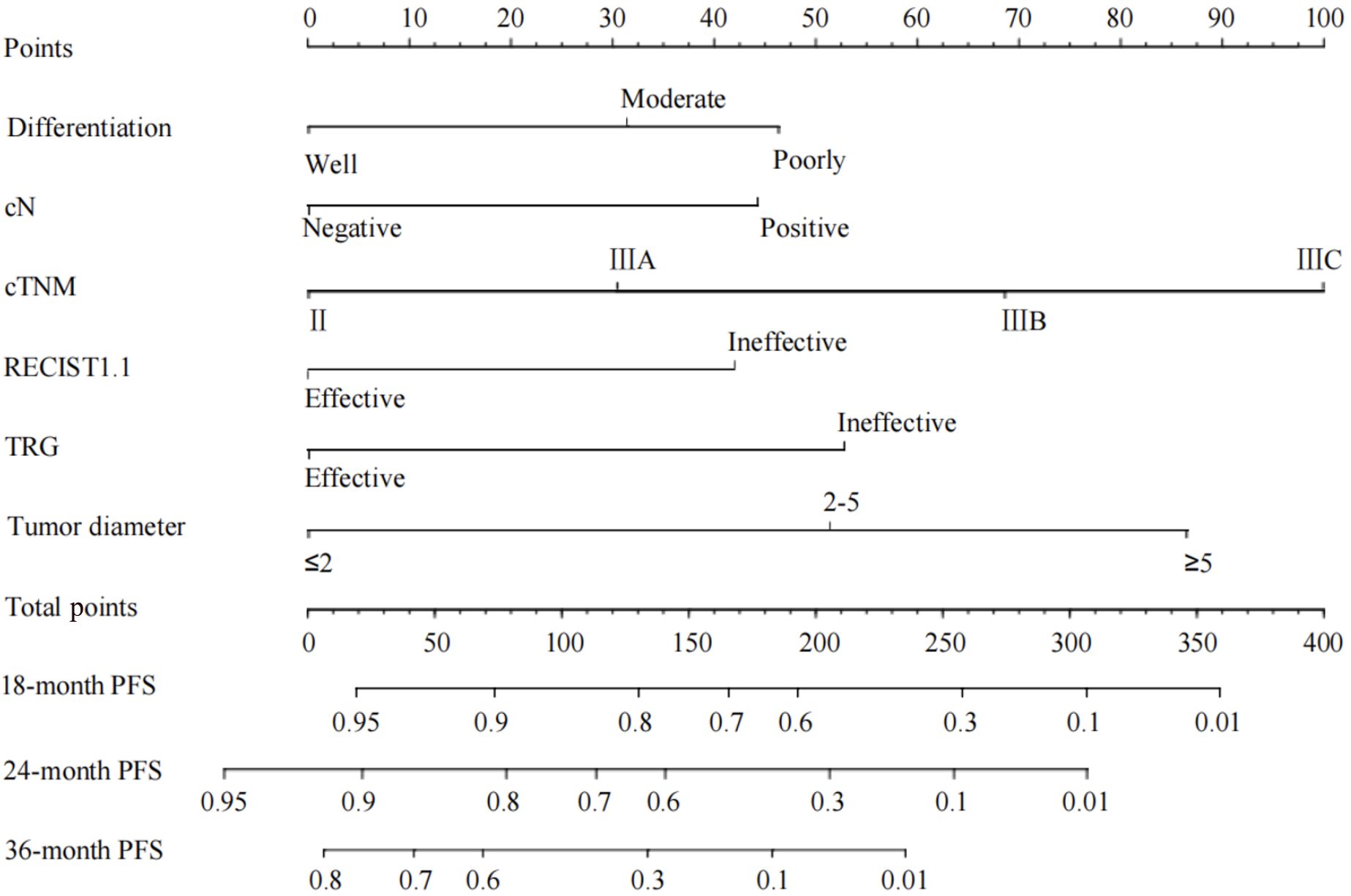
Figure 2 Progression-free survival nomogram.
PFS: Progression-free survival; RECIST: Response Evaluation Criteria in Solid Tumors; TRG: Tumor regression grade.
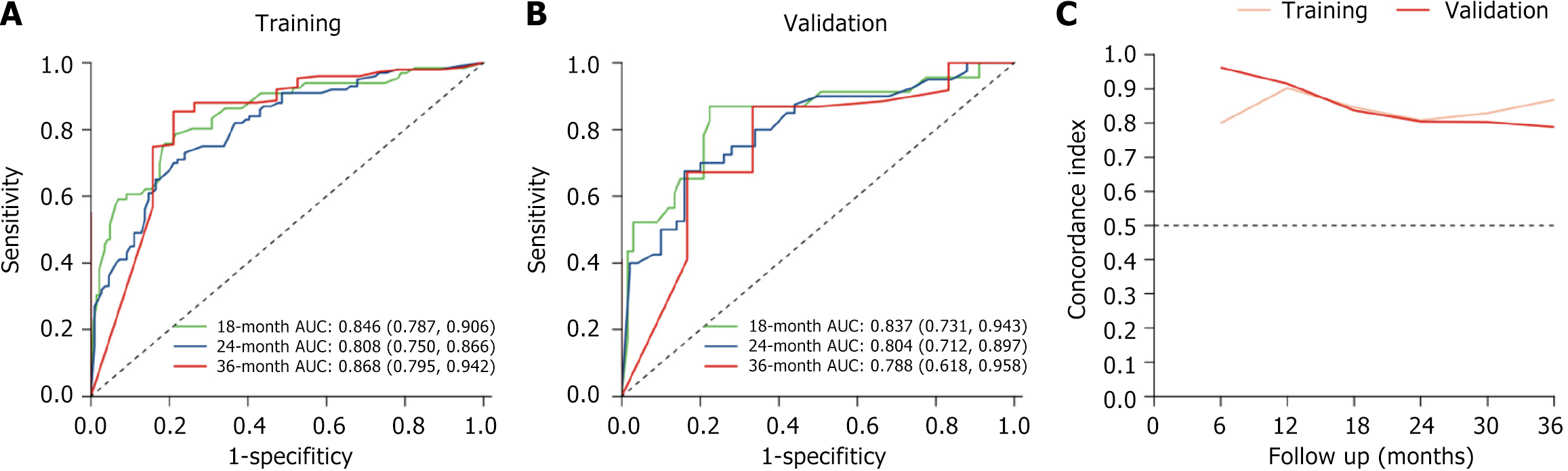
Figure 3 Progression-free survival receiver operating characteristic curve.
A: Training set; B: Validation set; C: Concordance index over time. AUC: Area under the curve.
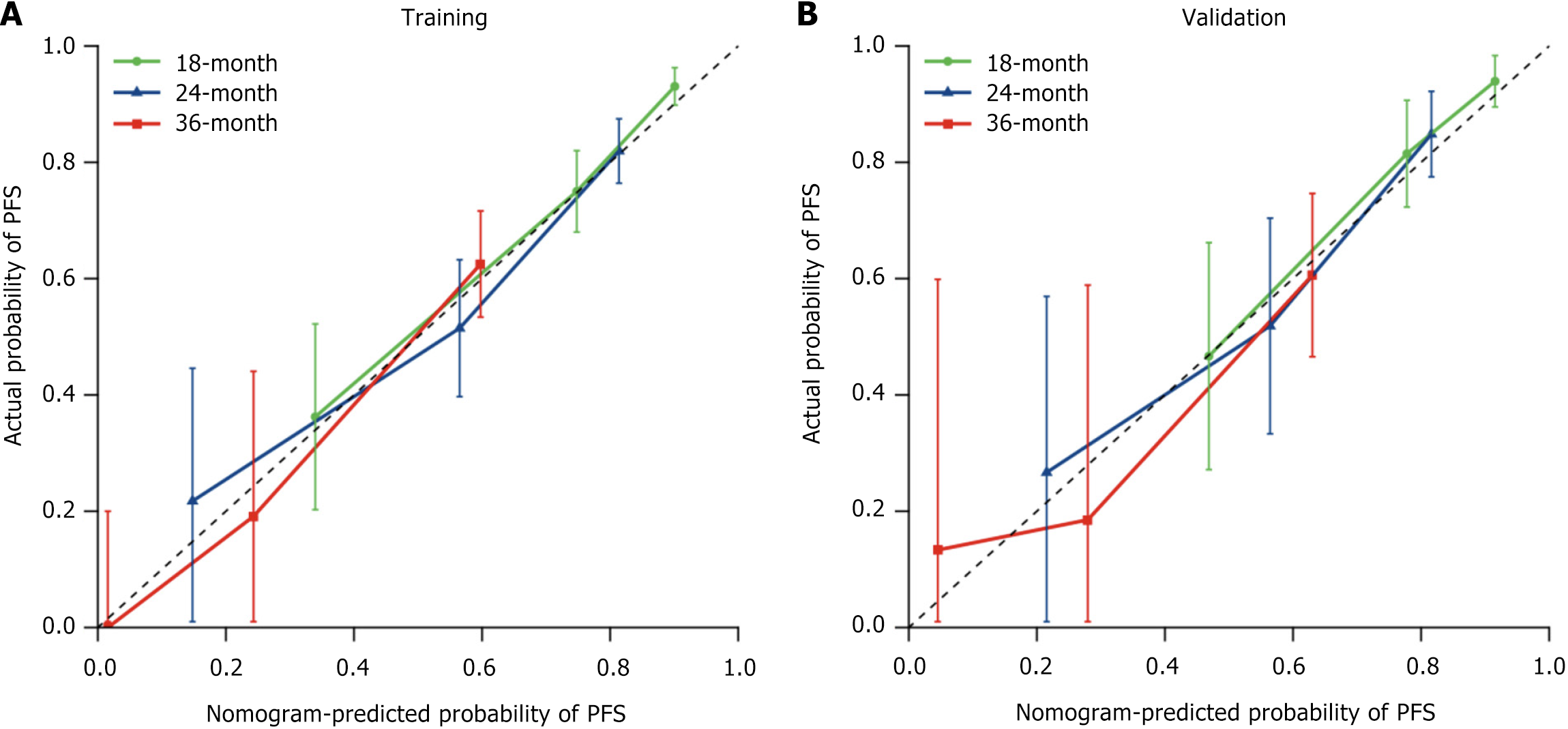
Figure 4 Calibration curve for progression-free survival.
A: Training set; B: Validation set. PFS: Progression-free survival.
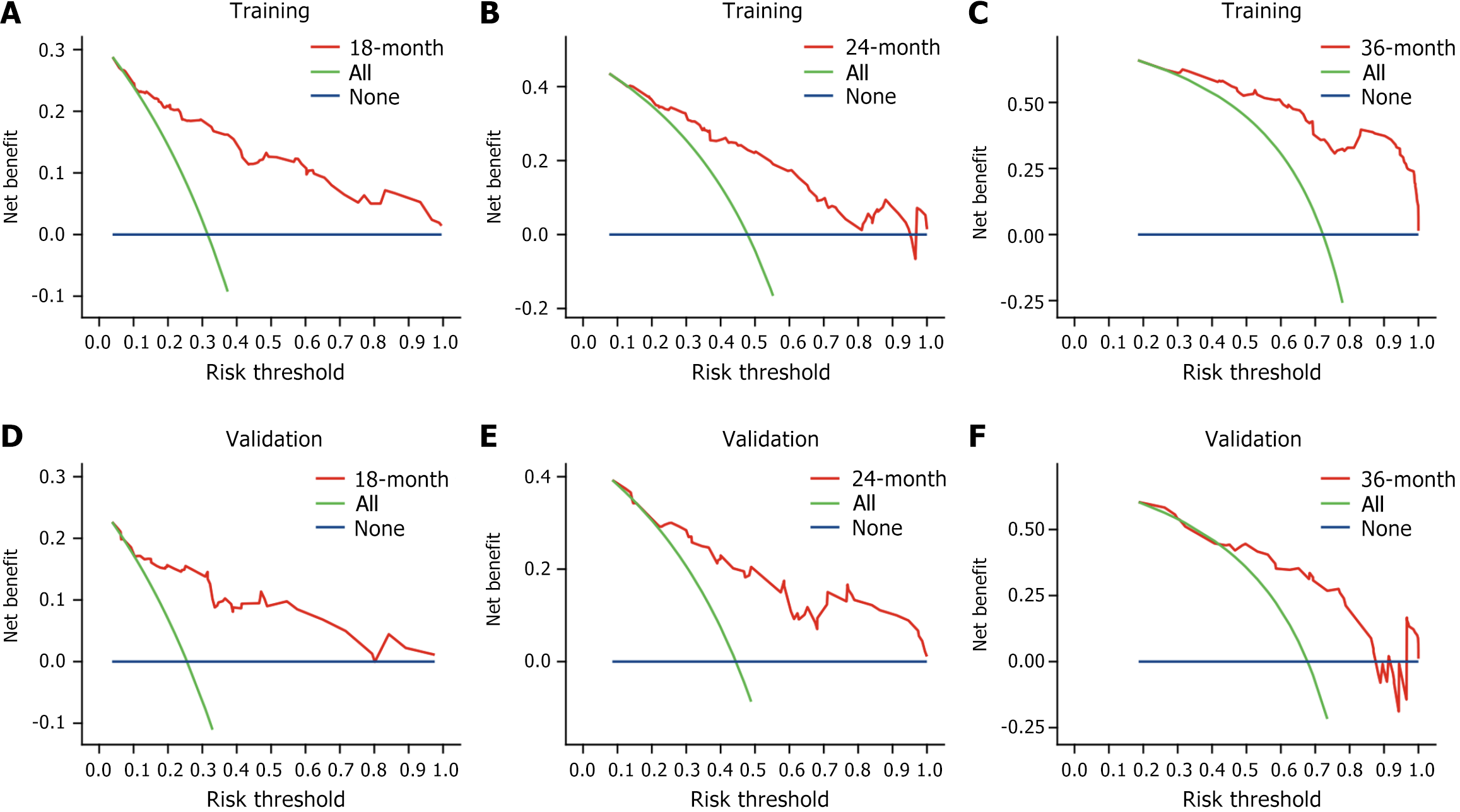
Figure 5 Decision curve analysis curves of progression-free survival.
A: 18-month progression-free survival (PFS), training set; B: 24-month PFS, training set; C: 36-month PFS, training set; D: 18-month PFS, validation set; E: 24-month PFS, validation set; F: 36-month PFS, validation set.
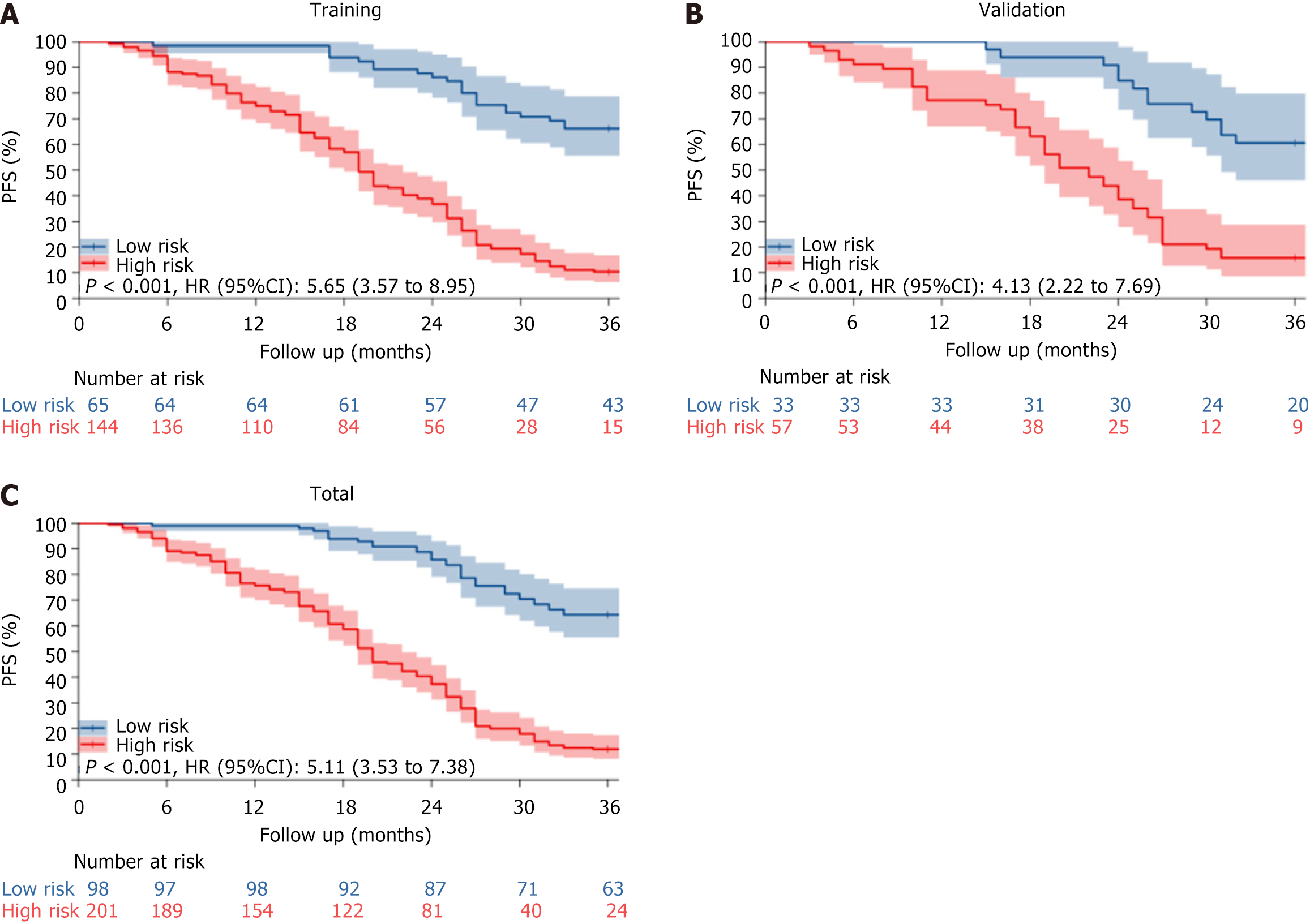
Figure 6 Kaplan-Meier survival curves for the verification of risk stratification.
A: Training set; B: Validation set; C: Total set. PFS: Progression-free survival.
- Citation: Wang YC, Zhang CG, Wang YW, Guo C, Pan T, Yu PJ, Cai BJ, Ding RH, Qiang JL, Deng CQ, Hu CH, Xu YH. SOX plus sintilimab vs P-SOX vs SOX as neoadjuvant therapy in advanced gastric cancer: Efficacy and safety. World J Gastrointest Oncol 2025; 17(8): 109646
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/109646.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.109646