Copyright
©The Author(s) 2023.
World J Gastrointest Oncol. Oct 15, 2023; 15(10): 1739-1755
Published online Oct 15, 2023. doi: 10.4251/wjgo.v15.i10.1739
Published online Oct 15, 2023. doi: 10.4251/wjgo.v15.i10.1739
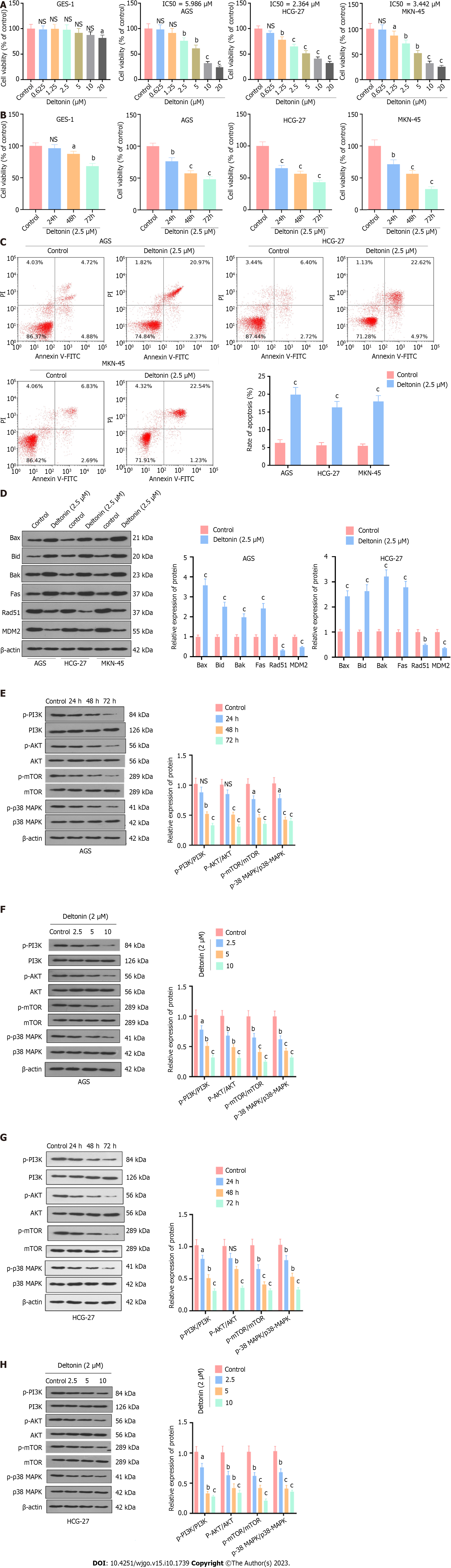
Figure 1 Deltonin inhibits gastric carcinoma cell proliferation and expedites their apoptosis.
A: Gastric carcinoma (GC) cell lines AGS, HGC-27, and MKN-45 were treated with deltonin (0 μM, 0.625 μM, 1.25 μM, 2.5 μM, 5 μM, 10 μM, and 20 μM) for 24 h. 3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium bromide (MTT) assay was used to examine cell viability; B: IC50 values of AGS, HGC-27, and MKN-45 cells treated with deltonin of different concentrations; C: Flow cytometry analysis of apoptosis of AGS, HGC-27, and MKN-45 cells treated with 2.5 μM deltonin for 24 h; D: Western blot analysis of expression of apoptosis-concerned proteins (Bax, Bak, Bid, and Fas) and DNA repair-associated proteins (Rad51 and MDM2) in GC cells; E: Western blot analysis of protein expression in AGS cells treated with 2.5 μM of deltonin for 0 h, 24 h, 48 h, and 72 h; F: Western blot analysis of protein expression in AGS cells treated with deltonin (0, 2.5, 5, and 10 μM) for 24 h; G: Western blot analysis of protein expression in HGC-27 cells treated with 2.5 μM of deltonin for 0 h, 24 h, 48 h, and 72 h; H: Western blot analysis of protein expression in HGC-27 cells treated with deltonin (0, 2.5, 5, and 10 μM) for 24 h; NS: P > 0.05, aP < 0.05, bP < 0.01, cP < 0.001 vs control group. n = 3. NS: No significance; PI3K: Phosphatidylinositol 3-kinase; AKT: Protein kinase B; mTOR: Mammalian target of the rapamycin; p38-MAPK: p38-mitogen-activated protein kinase.
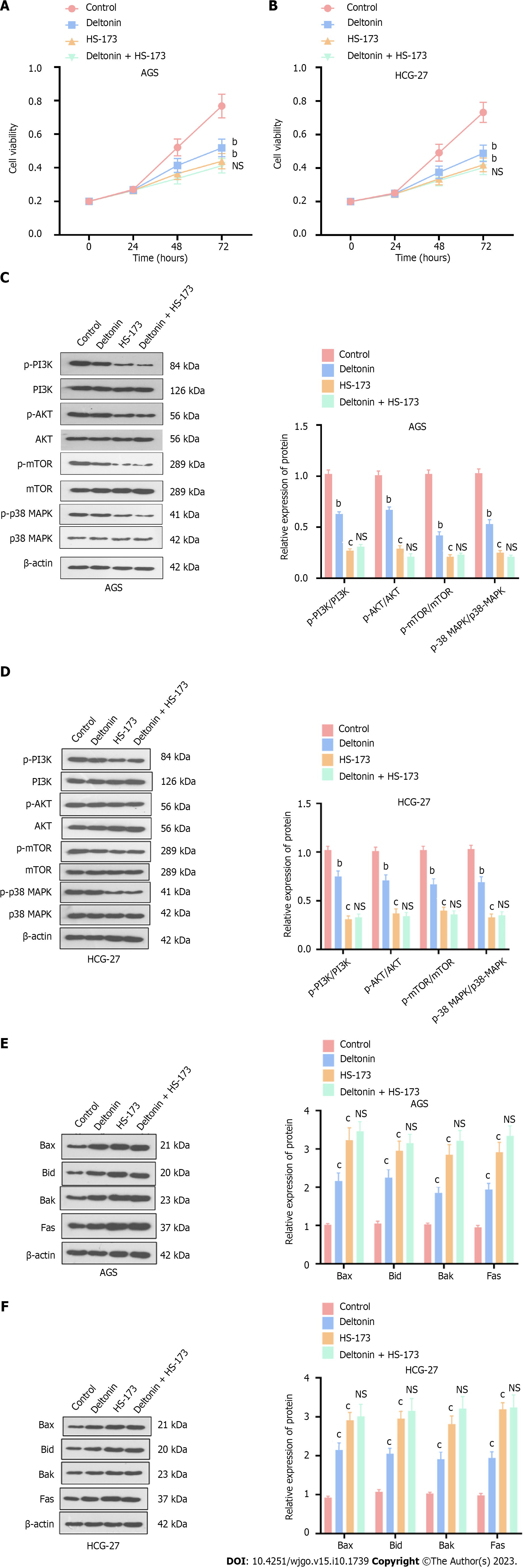
Figure 2 Deltonin attenuates gastric carcinoma cell viability by dampening the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of the rapamycin mitogen-activated protein kinase pathways.
HGC-27 and AGS cells were treated with 2.5 μM of deltonin and/or 0.8 nM of HS-173 for 24 h. A and B: 3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium bromide assay for cell viability examination; C and D: Western blot analysis of the profiles of apoptosis-correlated proteins; E and F: Western blot confirmation of the protein profiles of phosphatidylinositol 3-kinase/protein kinase B/mammalian target of the rapamycin p38-mitogen-activated protein kinase. bP < 0.01, cP < 0.001 vs control group; NS: P > 0.05 vs HS-173 group, n = 3. PI3K: Phosphatidylinositol 3-kinase; AKT: Protein kinase B; mTOR: Mammalian target of the rapamycin; p38-MAPK: p38-mitogen-activated protein kinase.
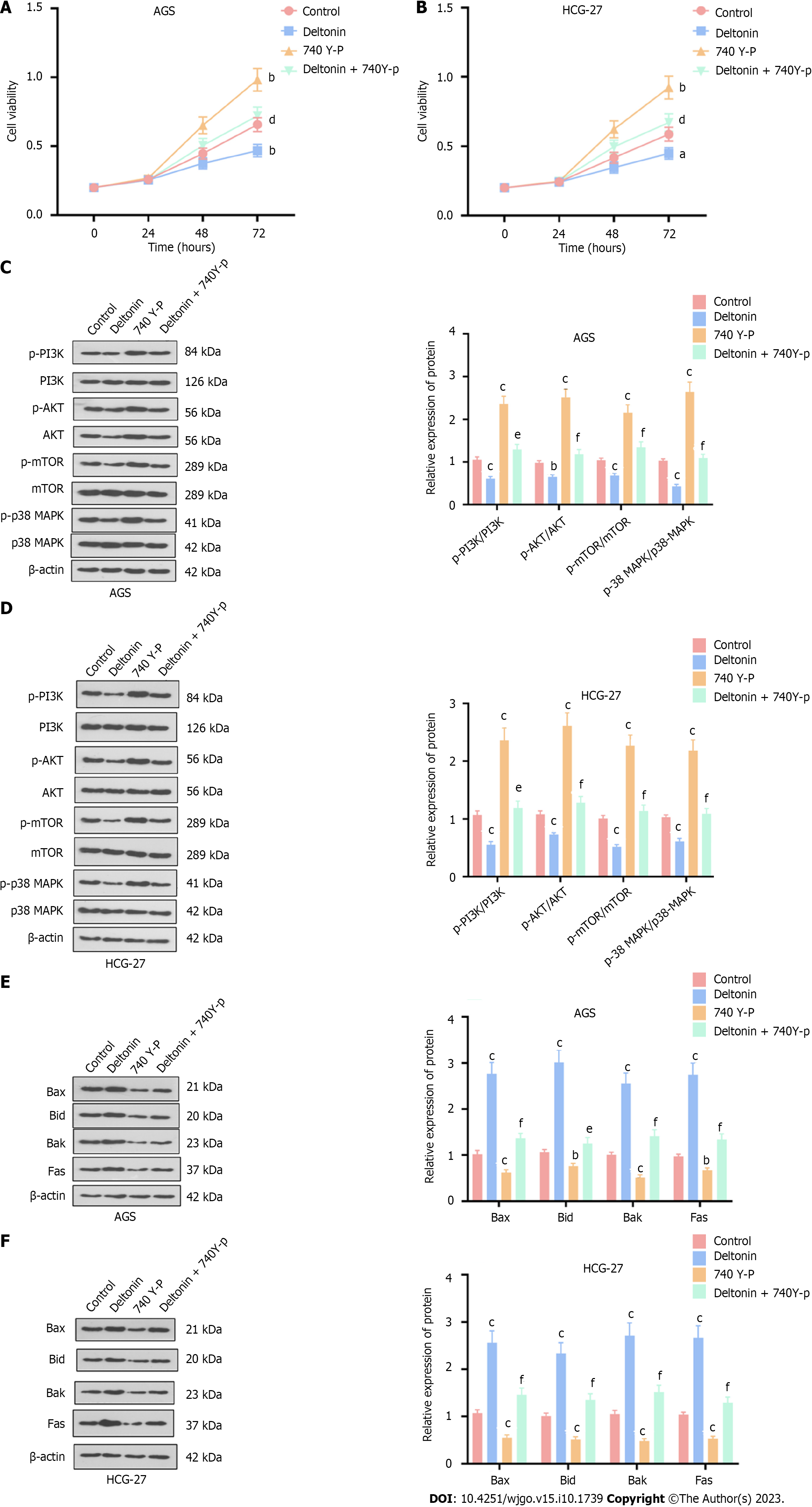
Figure 3 Influence of phosphatidylinositol 3-kinase/protein kinase B/mammalian target of the rapamycin p38-mitogen-activated protein kinase signaling pathway activation on the effects mediated by deltonin.
HGC-27 and AGS cells were treated with 2.5 μM of deltonin and/or 20 μM of phosphatidylinositol 3-kinase activator 740 Y-P for 24 h. A and B: 3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium bromide assay for cell viability; C and D: Western blot verification of the profiles of apoptosis-concerned proteins; E and F: Western blot determination of the protein profiles of phosphatidylinositol 3-kinase/protein kinase B/mammalian target of the rapamycin and p38-mitogen-activated protein kinase. aP < 0.05, bP < 0.01, cP < 0.001 vs control group; dP < 0.05, eP < 0.01, fP < 0.001 vs 740 Y-P group, n = 3. PI3K: Phosphatidylinositol 3-kinase; AKT: Protein kinase B; mTOR: Mammalian target of the rapamycin; p38-MAPK: p38-mitogen-activated protein kinase.
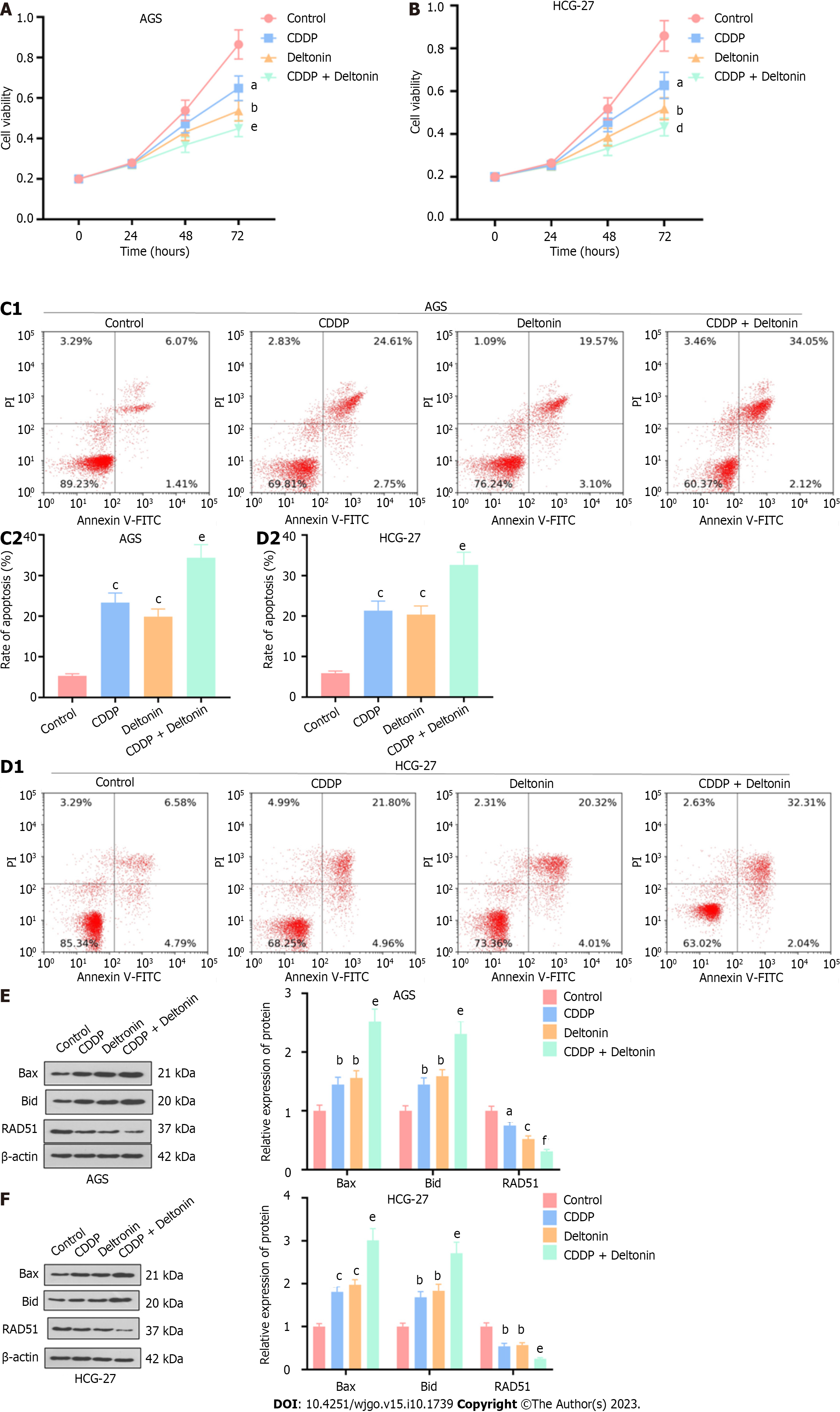
Figure 4 Deltonin enhances the chemosensitivity of gastric carcinoma cells to cisplatin.
AGS and HGC-27 gastric carcinoma cells were treated with 2.5 μM of deltonin or 5 μg/mL of cisplatin or deltonin (1.25 μM) plus cisplatin (2.5 μg/mL) for 24 h. A and B: 3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium bromide assay for examination of cell viability; C and D: Flow cytometry analysis of cell apoptosis; E and F: Western blot analysis of expression of apoptosis-correlated proteins (Bax and Bid) and the DNA repair-associated protein Rad51. aP < 0.05, bP < 0.01, cP < 0.001 vs control group; dP < 0.01, eP < 0.01, fP < 0.001 vs cisplatin group, n = 3. CDDP: Cisplatin.
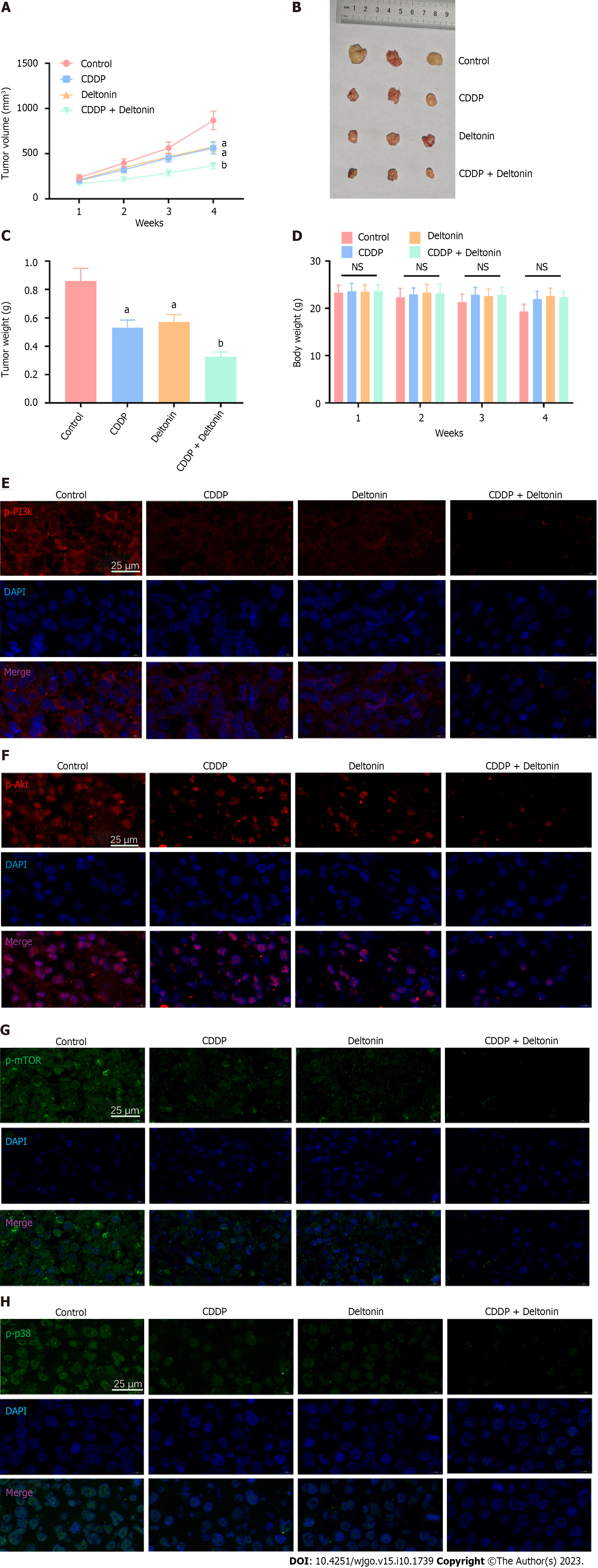
Figure 5 Deltonin augmentes the chemosensitivity to cisplatin in vivo by dampening the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of the rapamycin and mitogen-activated protein kinase signaling pathways.
Tumor-bearing mice were treated with saline, deltonin (50 mg/kg), and cisplatin (3 mg/kg) or deltonin (25 mg/kg) + cisplatin (1.5 mg/kg). A: The tumor volume was calculated during the 28 d; B and C: On the 28th d, the mice were sacrificed, the tumor images were taken, and the tumor weight was gauged; D: The body weight of the nude mice in different groups was figured out; E-H: Immunofluorescence measurement of the levels of phosphorylated phosphatidylinositol 3-kinase/protein kinase B/mammalian target of the rapamycin and p38-mitogen-activated protein kinase in the tumor tissues. NS: P > 0.05, aP < 0.01 vs sham group, bP < 0.01 vs cisplatin group, n = 3. NS: No significance; PI3K: Phosphatidylinositol 3-kinase; AKT: Protein kinase B; mTOR: Mammalian target of the rapamycin; DAPI: 4’,6-diamidino-2-phenylindole; CDDP: Cisplatin.
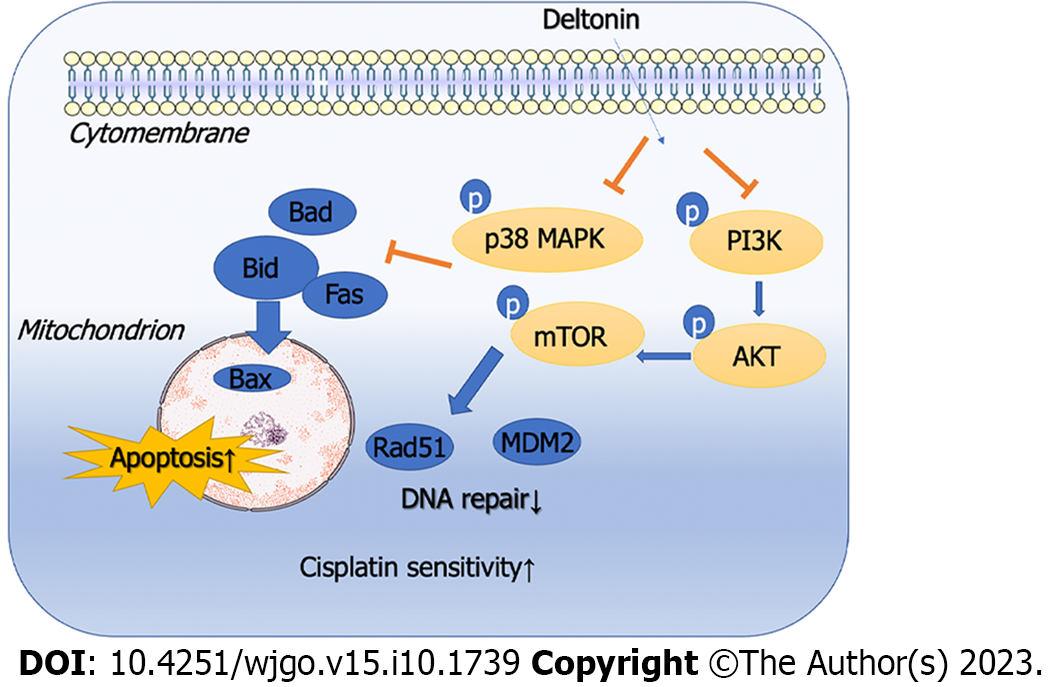
Figure 6 Mechanism diagram.
Deltonin bolsteres the apoptosis of gastric cancer cells and enhances their chemosensitivity to cisplatin via the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of the rapamycin and mitogen-activated protein kinase signaling pathways. PI3K: Phosphatidylinositol 3-kinase; AKT: Protein kinase B; mTOR: Mammalian target of the rapamycin; p38-MAPK: p38-mitogen-activated protein kinase.
- Citation: Yang L, Liu YN, Gu Y, Guo Q. Deltonin enhances gastric carcinoma cell apoptosis and chemosensitivity to cisplatin via inhibiting PI3K/AKT/mTOR and MAPK signaling. World J Gastrointest Oncol 2023; 15(10): 1739-1755
- URL: https://www.wjgnet.com/1948-5204/full/v15/i10/1739.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i10.1739