Copyright
©The Author(s) 2021.
World J Gastrointest Oncol. Sep 15, 2021; 13(9): 1144-1156
Published online Sep 15, 2021. doi: 10.4251/wjgo.v13.i9.1144
Published online Sep 15, 2021. doi: 10.4251/wjgo.v13.i9.1144
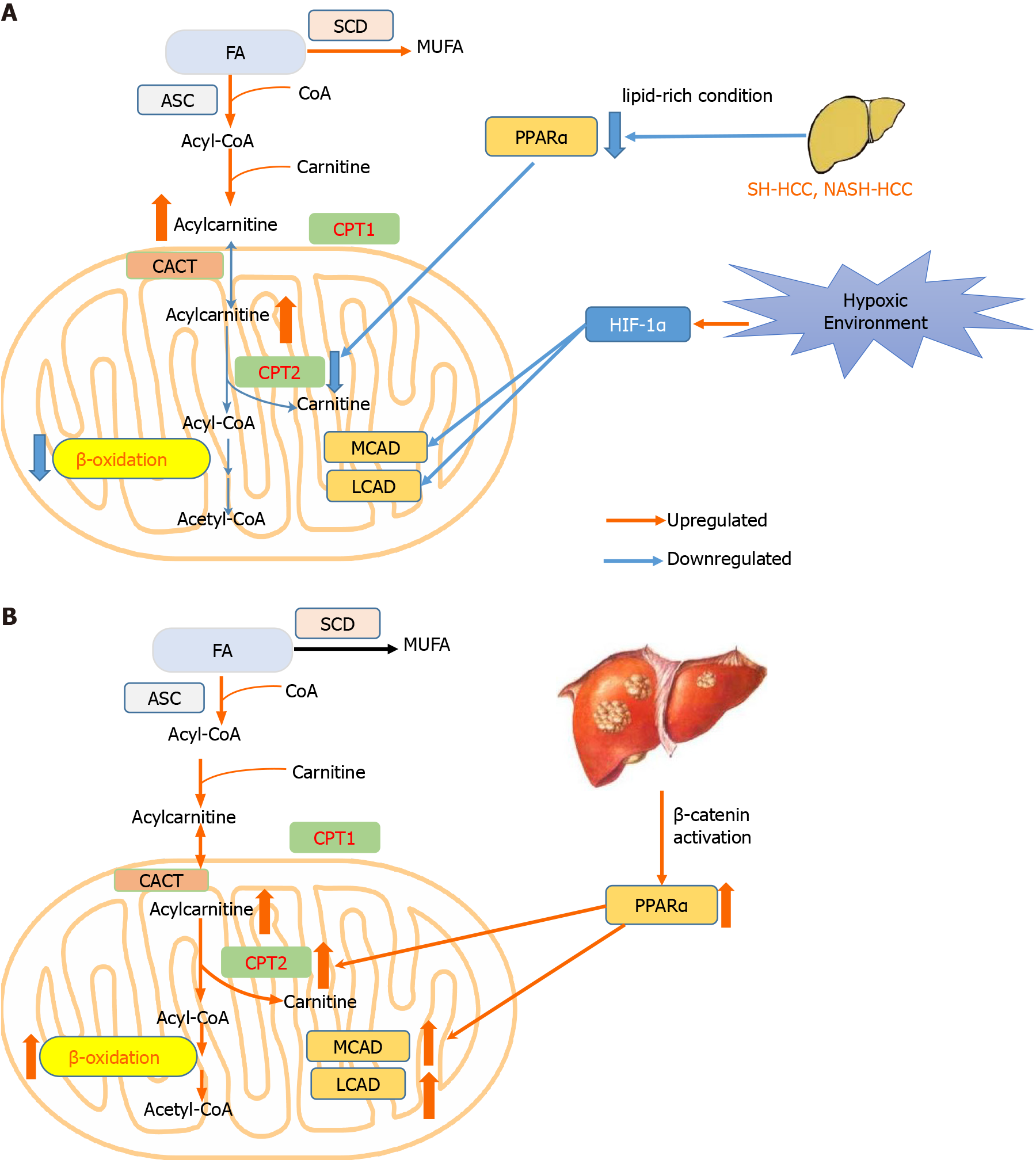
Figure 1 Hepatocellular carcinoma develops two distinct lipid metabolism patterns depending on the environment or genetic background of tumor cells.
A: In most conditions including hypoxia environment, and steatohepatitic or nonalcoholic steato-hepatitis-driven hepatocellular carcinoma (HCC), β-oxidation is repressed to avoid excessive oxidative damage; B: In HCC harboring β-catenin mutation, β-oxidation is promoted to trigger HCC. CACT: Carnitine–acylcarnitine translocase; CPT1: Carnitine palmitoyltransferase 1; CPT2: Carnitine palmitoyltransferase 2; FA: Fatty acid; HCC: Hepatocellular carcinoma; HIF-1α: Hypoxia inducible factor 1 subunit alpha; LCAD: Long-chain acyl-CoA dehydrogenases; MCAD: Medium-chain acyl-CoA dehydrogenases; MUFA: Monounsaturated fatty acid; PPARα: Peroxisome proliferator activated receptor alpha; SCD: Stearoyl-CoA desaturase.
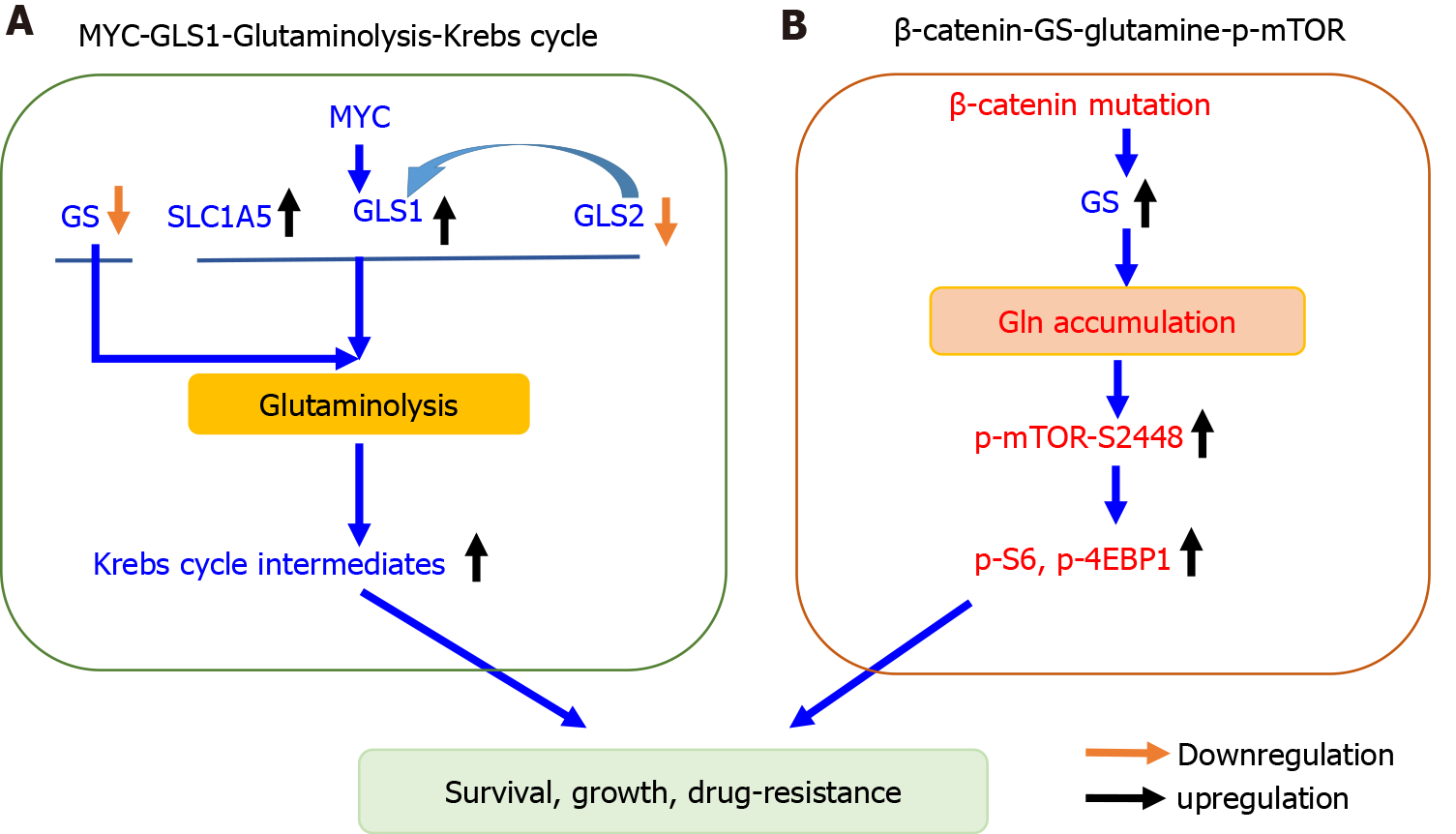
Figure 2 Two distinct principles governing glutamine metabolism in hepatocellular carcinoma.
A: Glutaminolysis in MYC-induced hepatocellular carcinoma (HCC) is enhanced via increasing transport and catabolism to fuel Krebs cycle; B: In HCC bearing β-catenin mutation, glutamine accumulation in response to glutamine synthetase upregulation further activates phosphorylated mammalian target of rapamycin. GLS1: Kidney-type glutaminase; GLS2: Liver type glutaminase; Gln: Glutamine; GS: Glutamine synthetase; SLC1A5: Solute-linked carrier family A1 member 5; p-4EBP1: Phosphorylated 4E-binding protein 1; p-mTOR: Phosphorylated mammalian target of rapamycin.
- Citation: Wu J, Xue R, Jiang RT, Meng QH. Characterization of metabolic landscape in hepatocellular carcinoma. World J Gastrointest Oncol 2021; 13(9): 1144-1156
- URL: https://www.wjgnet.com/1948-5204/full/v13/i9/1144.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i9.1144