Copyright
©The Author(s) 2018.
World J Gastrointest Oncol. May 15, 2018; 10(5): 108-114
Published online May 15, 2018. doi: 10.4251/wjgo.v10.i5.108
Published online May 15, 2018. doi: 10.4251/wjgo.v10.i5.108
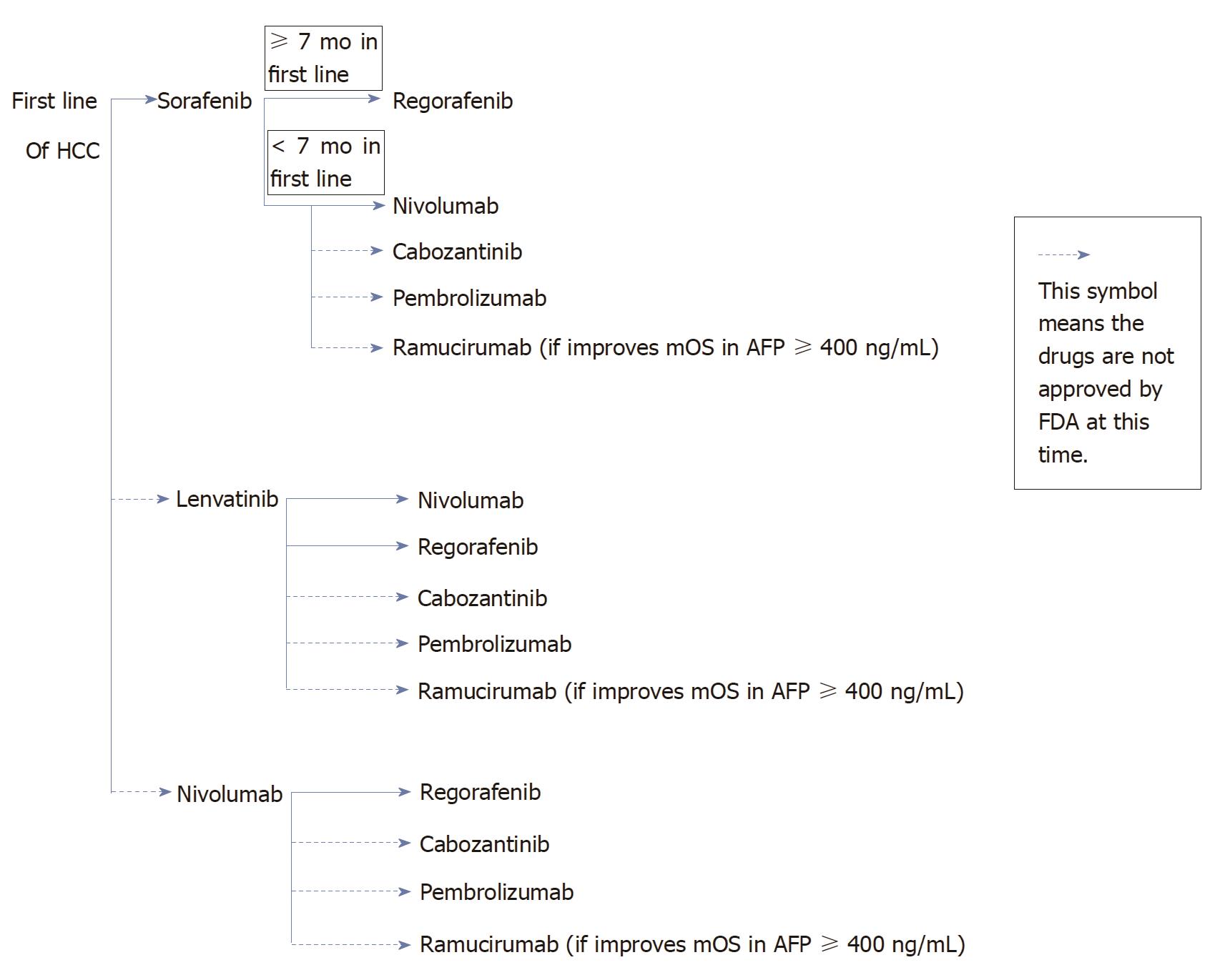
Figure 1 Potential sequencing treatment options in hepatocellular carcinoma.
The only food and drug administration (FDA) approved for first line systemic treatment for hepatocellular carcinoma (HCC) is sorafenib. If patients tolerate sorafenib well and could stay on therapy for at least 7 mo, regorafenib (FDA approved) would be a preferred second line option. If patients could not tolerate sorafenib well or received less than 7 mo of treatment with sorafenib, the next second line options will be nivolumab (FDA approved) and could be cabozantinib or pembrolizumab after get approval by FDA. Another potential first line option will be lenvatinib or nivolumab after get approval by FDA. If patients progress on lenvatinib, then second line options will be nivolumab, regorafenib, cabozantinib, pembrolizumab. For patients who progress on nivolumab, then second line options will be regorafenib, cabozantinib, pembrolizumab. Another possible option of second line treatment after patients progress after the above first line treatment could be ramucirumab if the phase III study shows improvement of mOS in HCC patients with AFP ≥ 400 ng/mL. FDA: Food and drug administration; mOS: Median overall survival; RR: Response rate.
- Citation: Contratto M, Wu J. Targeted therapy or immunotherapy? Optimal treatment in hepatocellular carcinoma. World J Gastrointest Oncol 2018; 10(5): 108-114
- URL: https://www.wjgnet.com/1948-5204/full/v10/i5/108.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i5.108