Published online Aug 25, 2016. doi: 10.4253/wjge.v8.i16.546
Peer-review started: March 27, 2016
First decision: May 17, 2016
Revised: June 1, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: August 25, 2016
In the last decade, the treatment of dysplastic Barrett’s esophagus has evolved into primarily endoscopic therapy. Many techniques have become well-established to destroy or remove the mucosal lining of Barrett’s esophagus. One of the newest therapies, cryospray ablation, has become a modality to treat both dysplastic Barrett’s esophagus as well as esophageal carcinoma. In endoscopic applications, the cryogen used is either liquid nitrogen or carbon dioxide which causes tissue destruction through rapid freeze-thaw cycles. Unlike other endoscopic ablation techniques, its unique mechanism of action and depth of tissue injury allow cryoablation to be used effectively in flat or nodular disease. It can be combined with other modalities such as endoscopic mucosal resection or radiofrequency ablation. Its esophageal applications stem well-beyond Barrett’s into ablation of early carcinoma, palliative debulking of advanced carcinoma and reduction of tumor ingrowth into stents placed for dysphagia. Although there are fewer reported studies of endoscopic cryoablation in the literature compared to other endoscopic ablation methods, emerging research continues to demonstrate its efficacy as a durable ablation technology with a variety of applications. The aim of this review is to examine the pathophysiology of endoscopic cryospray ablation, describe its outcomes in Barrett’s with dysplasia and esophageal carcinoma, and examine its role in other gastrointestinal applications such as hemostasis in the stomach and rectum.
Core tip: The current standard of care in treatment of dysplastic Barrett’s esophagus is endoscopic ablation. Cryospray ablation, the newest modality can achieve complete eradication of dysplasia and intestinal metaplasia in over 90% of patients. Unlike other endoscopic methods, its unique mechanisms and depth of injury enable successful ablation of early esophageal carcinoma, palliative debulking of advanced carcinoma and reduction of tumor ingrowth into stents. The applications of cryospray ablation beyond the esophagus include control of bleeding from gastric antral vascular ectasia, portal hypertensive gastropathy, and radiation proctitis. This modality continues to evolve as an important tool of therapeutic endoscopy.
- Citation: Sreenarasimhaiah J. Endoscopic applications of cryospray ablation therapy-from Barrett’s esophagus and beyond. World J Gastrointest Endosc 2016; 8(16): 546-552
- URL: https://www.wjgnet.com/1948-5190/full/v8/i16/546.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i16.546
The treatment of Barrett’s esophagus with dysplasia or intramucosal cancer has evolved in the past decade from a primarily surgical management into endoscopic therapy as the initial modality. Many endoscopic techniques have become well established to destroy or remove the mucosal lining of Barrett’s esophagus. One of the newest therapies, cryospray ablation, continues to evolve as a method for treatment of dysplastic Barrett’s esophagus as well as esophageal carcinoma. This technology was first introduced commercially to gastroenterologists in 2007 but has been based on methods used for over thirty years in fields such as dermatology, gynecology and urology to apply liquid nitrogen in the destruction of superficial lesions. In endoscopic applications, the cryogen used is either liquid nitrogen or carbon dioxide that are applied to cause rapid freezing and thawing of a target area with resulting tissue sloughing and subsequent growth of normal mucosa in its place. As one of the newest modalities for endoscopic ablation of Barrett’s, several studies have been reported and more are still underway to demonstrate its efficacy.
After its introduction in treatment of esophageal disease, endoscopic applications of cryospray ablation have continued into other areas of the gastrointestinal tract. FDA approval of the technology has been granted for a broad range indication of “cryosurgical tool for destruction of unwanted tissue in the field of general surgery, specifically for endoscopic applications”. With this charge, cryospray ablation has been applied in treatment of a variety of conditions such as palliation of obstructive esophageal cancer, gastric antral vascular ectasia and radiation proctitis. This review will describe the pathophysiology as well as the clinical applications of cryospray ablation in mainly the esophagus but also other areas of gastrointestinal endoscopy.
Introduced first in the 1960’s, liquid nitrogen cryosurgery was used to destroy lesions with applications of -20 °C. Since then, it has been shown that cellular apoptosis is achieved after reaching temperatures less than -50 °C[1]. Carbon dioxide cryospray ablation has been shown to reach temperatures of -78 °C while liquid nitrogen cryospray can reach temperatures of -196 °C. Freezing is usually performed at two to three cycles with applications ranging between 10 to 30 s each. The mechanism of action of thermal injury has two modalities. Flash freezing and thawing cycles that are repeatedly applied to a tissue causes immediate effects of slowing cellular metabolism and freezing intracellular water. Subsequently, ice formation results in disruption of cellular membranes and organelle dysfunction. Repeat freeze-thaw cycles add to the injury and cellular apoptosis ensues. The stromal intracellular collagen matrix remains intact and so the injury is not seen by endoscopic view during the immediate phase except for hyperemia of the mucosal surface. There is an immediate vasoconstriction followed later by vasodilation of the microcirculation and thus bleeding is not a major component of the early cellular injury. Delayed effects of the freeze-thaw cycles begin within hours to days with mucosal edema, anoxia, microthrombi formation, and apoptosis of the remaining surrounding tissue. This inflammatory response results in a cytokine mediated response involving Th1 cells following cellular apoptosis[2]. As the cellular scaffolding remains intact, healthy tissue regeneration follows over several weeks.
There are two main devices available commercially for the endoscopic application of cryospray ablation. First is liquid nitrogen cryospray known as Trufreeze (CSA Medical, Baltimore, MD) and the other is carbon dioxide cryospray known as Polar Wand (GI Supply, Camphill, PA). Another device that is currently undergoing clinical testing is the Coldplay Focal Cryoballoon Ablation System (C2 Therapeutics, Redwood City, CA).
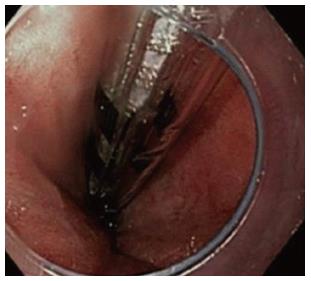
The Trufreeze liquid nitrogen system has become the most widely used of the endoscopic cryospray ablation systems with over 11000 treatments performed. This technology uses a generator that delivers cold liquid nitrogen at -196 °C through a flexible spray catheter with a low-flow (2-4 psi) continuous delivery in a noncontact method. Due to the potential for rapid expansion of the liquid nitrogen into 4 to 6 L of gas during a 20 s treatment, a multiport orogastric decompression catheter is placed with constant suction during the delivery of liquid nitrogen (Figure 1). The new generation flexible catheter permits retroflexion applications in the stomach or rectum up to 180°.
The treatment is performed with direct visualization of the mucosa to spray large areas of up to 4 cm length at a time. The depth of injury is dependent on the dosimetry of liquid nitrogen spray time. Traditional applications use 20 s cycles performed twice at each site for dysplastic Barrett’s mucosa. In the setting of intramucosal carcinoma, treatment may be performed for longer cycles of 30 s.
The depth of treatment is not limited to the mucosal surface. In contrast, radiofrequency ablation (RFA) has a set dosimetry and ablation depth of 500 microns which will not penetrate below the mucosal surface. Studies into the depth of penetration have been performed with cryospray liquid nitrogen application in the esophagus. Ribeiro prospectively studied a group of patients who were to undergo esophagectomy and applied liquid nitrogen cryospray preoperatively. Using 20 s cycles twice in the same area showed that 93% of patients had cell necrosis into the submucosal layer[3]. If applied in the same area long-enough, esophageal perforation can result as a combination of deep ablation as well as increased esophageal wall tension from rapid gas expansion[4].
This technology uses a through-the-scope spray catheter to deliver compressed liquid carbon dioxide that rapidly expands during spray and reaches -78 °C as it exits the catheter. This temperature has been shown to be effective for inducing cellular apoptosis. It has been given FDA clearance for use throughout the GI tract for focal mucosal ablation. Due to the lower flow volume compared to the liquid nitrogen cryospray, a separate decompression catheter is not required. However, a suction channel is directly connected to the spray catheter as it requires a flow of 6 to 8 L/min CO2 to achieve a temperature of less than -70 °C. Rapid expansion from a high pressure liquid to a low pressure gas results in a significant drop in temperature as explained by the Joule-Thomson effect.
While the vast majority of endoscopic ablation of Barrett’s mucosa is performed by either RFA or spray cryotherapy, both have their limitations such as the need for sizing, multiple deployment steps, large consoles, and decompression catheter placement. The new Coldplay Focal Cryoballoon Ablation System aims to overcome some of these restrictions. It uses a combination of an inflatable balloon passed through the accessory channel of the endoscope and applies liquid carbon dioxide. The balloon is highly compliant and conforms to the esophageal lumen without excessive tension of the esophageal wall and does not require special decompression catheters. Unlike the inflatable balloon device of RFA, pretreatment sizing is not required with this system. The device has received United States FDA 510 (k) clearance and is undergoing clinical study.
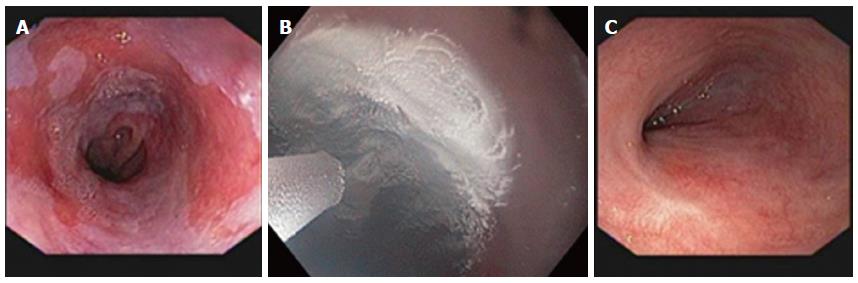
Endoscopic ablation of dysplastic Barrett’s has become well established and validated by many studies within the past decade. As per AGA guidelines, endoscopic ablation of Barrett’s esophagus is indicated in high-grade dysplasia (HGD) and possibly persistent low-grade dysplasia (LGD) but not in nondysplastic Barrett’s epithelium[5]. The ACG practice guidelines of 2015 confirm these same recommendations and also recommend endoscopic mucosal resection (EMR) initially for nodules followed later by endoscopic ablation therapy[6]. The vast majority of recent studies have examined a different modality, RFA. In a meta-analysis of 18 studies in 3802 patients examining RFA for Barrett’s, the results show a complete response in eradication of intestinal metaplasia of 78% and overall dysplasia of 91%[7]. However, there are several important studies examining the efficacy of cryospray therapy. Most of these are in regard to liquid nitrogen therapy and show results that are equal to the outcomes of RFA (Figure 2).
Most patients undergoing esophageal cryoablation will require treatment in multiple sessions that are usually separated by 6 to 8 wk intervals to allow for healing of the mucosa. Contraindications to treatment include mucosal breaks such as active esophagitis, erosions, and ulcerations seen at the time of endoscopy due to potential perforation. A tight stricture of the esophagus through which a decompression catheter as well as endoscopic spray catheter cannot both be placed together will also preclude safe treatment. Altered anatomy such as bariatric surgery is a contraindication for therapy due to difficulty in ventilating gas safely from the gastrointestinal tract. The safety of this procedure has been shown in several studies below.
Shaheen et al[6] examined 98 patients with HGD with a mean age of 65.4 years and mean Barrett’s length of 5.3 cm. In this group of 87% males, an average of 3.4 treatments per patient was performed with liquid nitrogen cryospray to achieve complete ablation. HGD was eradicated in 97% of all patients while 87% had complete eradication of all dysplasia. No perforations occurred and a stricture rate of 3% was identified and treated easily with endoscopic balloon dilation in all cases[8]. Additionally, this study showed a 1%-2% incidence of chest discomfort that required outpatient narcotic use. This is in contrast to RFA therapy which has been shown to have a significantly higher incidence of chest discomfort sometimes requiring hospitalization up to day 8 following the procedure compared to a sham treatment group and an overall esophageal stricture rate of 6%[9].
Greenwald et al[10] further demonstrated in a group of 7 patients with stage I esophageal adenocarcinoma that complete response was achieved in 100% with liquid nitrogen cryospray ablation alone. The same group demonstrated recently in a cohort of 33 patients followed long-term for at least 24 mo that a durable response can be achieved. Complete response for HGD was 97% and complete response for intestinal metaplasia was 87% at 24 mo[11].
Recurrence of disease after cryoablation for HGD achieved a complete response has also been evaluated. Halsey et al[12] prospectively examined a group of 36 patients who had HGD and underwent liquid nitrogen cryospray therapy. In 11 (30%) patients, recurrent disease was identified at a median of 6.5 mo. In 70% of these patients, recurrences occurred below the neosquamocolumnar junction including a variety of histology such as HGD, LGD, and intestinal metaplasia. In one patient, recurrent disease was esophageal carcinoma within the previously treated esophagus. This patient as well as a total of 33 patients (92%) ultimately achieved complete response to retreatment with cryotherapy[12]. This demonstrates the importance of follow-up surveillance biopsies after completion of cryoablation therapy not only within the previously treated esophagus but also at the gastric cardia immediately below the squamocolumnar junction.
While the cryoballoon focal ablation system is not commercially available, it has been studied for feasibility and efficacy in ablation of Barrett’s mucosa. In a prospective, non-randomized trial of 39 patients, 62 ablations were performed between 6-10 s. No adverse events occurred and no strictures resulted from the treatment. Mild pain was noted in 27% of patients. Full squamous regeneration was noted in 47 treated areas (60 % of 6-s cycles, 82% of 8-s cycles, and 100% of 10-s areas). Long-term follow-up of these patients as well as durable responses for HGD or LGD is being examined in ongoing studies[13].
The presentation of esophageal neoplasia can range from a small nodule or flat area of intramucosal carcinoma to a large bulky obstructing tumor with ulceration, bleeding and metastases. The standard of care in management of nodular mucosa within Barrett’s esophagus is endoscopic mucosal resection. However, larger flat areas of intramucosal cancer may be difficult to treat with EMR alone as well as difficulty with overlapping areas for complete treatment[14]. The combination of cryoablation therapy with EMR has been reported to be effective.
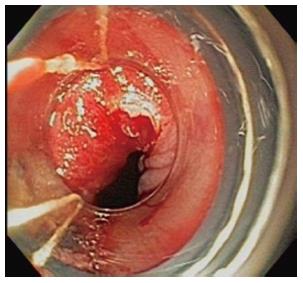
Liquid nitrogen cryoablation has been performed safely prior to and following EMR, as well as during the same session[15]. As described above, cryoablation causes destruction of cellular contents but maintains the intracellular collagen matrix. The structural injury is delayed and enables further therapy to the treated tissue. This may explain how this treatment can be easily combined with endoscopic mucosal resection which alone may be challenging if there is scarring or adherence of esophageal wall layers (Figure 3).
While the data for liquid nitrogen as the cryogen for ablation of esophageal neoplasia seems promising, the use of carbon dioxide has not been shown to achieve similar results. In a recent study of 30 patients with Barrett’s and early neoplasia, CO2 cryoablation therapy was performed. In 9 patients, nodular areas were first treated with EMR. With a mean of 2.5 cryoablation sessions and a six-month follow up of 10 patients, early termination of the study occurred due to the disappointing results with eradication of dysplasia in only 44% and persistence of neoplasia in a large portion. This study suggests that CO2 cryoablation combined with EMR may not be an effective modality for treatment of Barrett’s associated neoplasia[16].
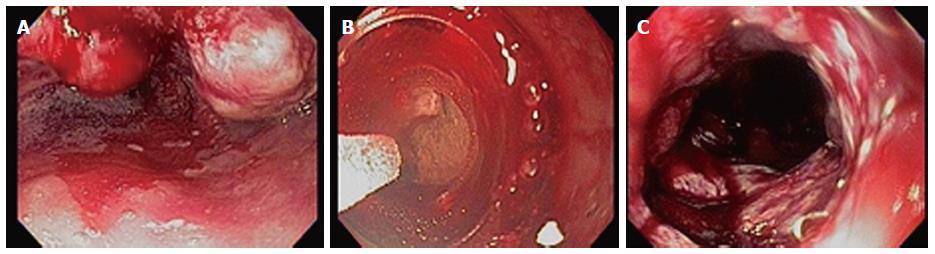
Debulking of esophageal cancer for palliation of swallowing has been shown to be feasible (Figure 4). Tumor ingrowth into a palliative metal esophageal stent can also be treated[17]. No outcome studies of cryoablation for palliation of dysphagia have been published. In a recent report, a 63-year-old patient with esophageal squamous cell carcinoma who had recurrence of disease had tumor ingrowth at the ends of a previously placed metal stent resulting in dysphagia. Liquid nitrogen cryotherapy was used to recanalize the lumen of the metal stent successfully[18]. Cash et al[19] reported the first application of liquid nitrogen cryotherapy for recurrent esophageal squamous cell cancer that occurred 3 years after definitive chemotherapy. This patient was disease-free at two year follow-up. In another study, 7 patients with superficial esophageal adenocarcinoma had complete response to cryoablation therapy in all patients at a range of follow-up between 3 to 18 mo[10]. Greenwald et al[20] reported liquid nitrogen cryoablation treatment of 79 patients with adenocarcinoma (tumor stage included T1-60, T2-16, and T3/4-3). Complete response of intraluminal disease was achieved in 61% and in 75% of patients with intramucosal (T1) disease. Mean follow up was 10.6 mo overall and 11.5 mo for T1 disease.
Hemostasis of bleeding from advanced esophageal carcinoma has been shown to be feasible with endoscopic cryoablation. Shah et al[21] reported a case of a 62-year-old male with locally advanced unresectable adenocarcinoma of the esophagus with bleeding that did not respond to chemotherapy, radiation therapy, brachytherapy, or photodynamic therapy. Liquid nitrogen cryospray ablation was used with three 20 s applications and resulted in reduction of blood transfusions from 30 units over the preceding two weeks to one unit over the following two weeks. Immediate post-procedural hemostasis as well as a durable response was noted.
Gastric antral vascular ectasia (GAVE) is a well-recognized entity that causes chronic blood loss from the upper gastrointestinal tract. It is often associated with connective tissue disease, liver cirrhosis, and renal failure but may also be of idiopathic origin[22]. The most common type is also known as “water-melon stomach” due to its classic endoscopic appearance of striped mucosa radiating from the pylorus. The other type is characterized by diffuse punctate erythematous angiomas of the antrum that is often associated with portal hypertension and cirrhosis[23].
Traditional endoscopic therapies of GAVE include the gold-standard of argon plasma coagulation (APC) which is a non-contact thermal method that can cause mucosal ablation and perhaps deeper injury as well. It often requires multiple sessions and has been shown to be very effective in mild to moderate disease but bleeding may be refractory in underlying cirrhosis or severe mucosal involvement[24]. Other treatments that have been tried with some limited success include thermal heater probe therapy, YAG laser ablation, and band ligation. In small studies, RFA has recently been demonstrated to be effective in reducing the blood transfusion requirements within the 6 mo period following treatment for those patients with GAVE refractory to initial APC therapy[25,26].
Cryospray ablation can be used as a secondary line of endoscopic therapy for refractory GAVE as it may be able to cover a larger area through spray therapy than other modalities. However, it is limited by gas flow and potential air entrapment in the small intestine. While it has been described, very few studies are available to show its efficacy. Kantsevoy showed in a pilot study of 7 patients with GAVE and recurrent bleeding that nitrous oxide cryoablation was effective in 71% for cessation of bleeding[27]. Carbon dioxide cryoablation was examined in a study of 12 patients with refractory GAVE and significant iron-deficiency anemia. All of these patients had undergone APC therapy with a median of 6 sessions. In this group, 50% achieved complete response with a mean of 3 sessions of cryoablation and 50% had a partial response manifest by incomplete ablation but stable hemoglobin. The entire group had a mean increase in hemoglobin from 9.9 to 11.3 g/dL. No adverse events were noted in any patient[28]. Liquid nitrogen spray cryotherapy has also been examined in treatment of GAVE and portal hypertensive gastropathy with refractory bleeding. It was shown to be very effective in cessation of bleeding from portal hypertensive gastropathy that did not respond to either APC or transjugular intrahepatic portosystemic shunt placement[29].
Chronic radiation proctitis occurs in up to 15% of patients within months to even decades following radiation therapy for pelvic malignancies. Most patients will present with recurrent rectal bleeding and often have rectal pain and tenesmus. Traditional medical therapies for radiation proctitis include enemas with salicylates, sucralfate, and corticosteroids which may help short-term symptoms but have not been shown to have long-term effects[30]. Endoscopic therapy has traditionally included APC which is very effective in mild to moderate radiation proctitis requiring several sessions to achieve ablation. In more severe mucosal damage, refractory proctitis is present in up to 50% of patients[31]. Recent reports demonstrate RFA with the Halo90 system to be effective in moderate radiation proctitis with 1 to 2 sessions and effective control of lower gastrointestinal bleeding[32].
While both APC and RFA require a contact method of treatment and may be limited by blood or tissue adherence, cryoablation has been used as noncontact application for treatment of chronic radiation proctitis. In a recent study, treatment was applied for 5 s applications to reduce the risk of proximal gas entrapment and perforation. Patients required between 1 and 4 sessions. In all patients, significant response was seen in endoscopic score of proctitis, and improvement in rectal pain and bleeding[33].
Cryoablation therapy has become well-established as a modality for treatment of dysplastic Barrett’s esophagus. Due to its potential for deeper tissue injury, it has evolved into successful applications of ablation of nodular Barrett’s and early esophageal carcinoma with or without combined EMR therapy. This modality also serves as an alternative when other endoscopic ablation modalities such as RFA or APC are refractory or contraindicated in high risk settings such as chronic anticoagulation, implanted cardiac defibrillators, esophageal strictures, radiation therapy, or within esophageal stents. Other applications of cryoablation in the stomach or rectum to treat bleeding angioectasia have been shown to be feasible. As the newest modality of endoscopic mucosal ablation, more efficacy studies as well as novel applications within the gastrointestinal tract are continuing to emerge, ensuring that cryotherapy will remain an important tool for therapeutic endoscopy.
Manuscript source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: United States
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Geraci G, Guo YM S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Gage AA, Baust JM, Baust JG. Experimental cryosurgery investigations in vivo. Cryobiology. 2009;59:229-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 779] [Cited by in F6Publishing: 638] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 3. | Ribeiro A, Bejarano P, Livingstone A, Sparling L, Franceschi D, Ardalan B. Depth of injury caused by liquid nitrogen cryospray: study of human patients undergoing planned esophagectomy. Dig Dis Sci. 2014;59:1296-1301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Dumot JA, Vargo JJ, Falk GW, Frey L, Lopez R, Rice TW. An open-label, prospective trial of cryospray ablation for Barrett’s esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc. 2009;70:635-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 373] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 6. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 990] [Cited by in F6Publishing: 982] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 7. | Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1245-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 8. | Shaheen NJ, Greenwald BD, Peery AF, Dumot JA, Nishioka NS, Wolfsen HC, Burdick JS, Abrams JA, Wang KK, Mallat D. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high-grade dysplasia. Gastrointest Endosc. 2010;71:680-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 9. | Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277-2288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1046] [Cited by in F6Publishing: 914] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 10. | Greenwald BD, Dumot JA, Horwhat JD, Lightdale CJ, Abrams JA. Safety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Dis Esophagus. 2010;23:13-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Gosain S, Mercer K, Twaddell WS, Uradomo L, Greenwald BD. Liquid nitrogen spray cryotherapy in Barrett’s esophagus with high-grade dysplasia: long-term results. Gastrointest Endosc. 2013;78:260-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Halsey KD, Chang JW, Waldt A, Greenwald BD. Recurrent disease following endoscopic ablation of Barrett’s high-grade dysplasia with spray cryotherapy. Endoscopy. 2011;43:844-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Schölvinck DW, Künzli HT, Kestens C, Siersema PD, Vleggaar FP, Canto MI, Cosby H, Abrams JA, Lightdale CJ, Tejeda-Ramirez E. Treatment of Barrett’s esophagus with a novel focal cryoablation device: a safety and feasibility study. Endoscopy. 2015;47:1106-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Mino-Kenudson M, Brugge WR, Puricelli WP, Nakatsuka LN, Nishioka NS, Zukerberg LR, Misdraji J, Lauwers GY. Management of superficial Barrett’s epithelium-related neoplasms by endoscopic mucosal resection: clinicopathologic analysis of 27 cases. Am J Surg Pathol. 2005;29:680-686. [PubMed] [Cited in This Article: ] |
| 15. | Hussain Z, Fukami N, Smith M, Sreenarasimhaiah J, Kaul V, Kothari S, Greenwald BD, Shaheen NJ. Safety and Efficacy of Same Session Spray Cryotherapy and Endoscopic Mucosal Resection for Barrett’s Esophagus and Early Esophageal Neoplasia: a Multicenter Experience. Gastrointest Endosc. 2015;81 Suppl:AB508. [DOI] [Cited in This Article: ] |
| 16. | Verbeek RE, Vleggaar FP, Ten Kate FJ, van Baal JW, Siersema PD. Cryospray ablation using pressurized CO2 for ablation of Barrett’s esophagus with early neoplasia: early termination of a prospective series. Endosc Int Open. 2015;3:E107-E112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Barthel JS, Kucera S, Harris C, Canchi D, Hoffe S, Meredith K. Cryoablation of persistent Barrett’s epithelium after definitive chemoradiation therapy for esophageal adenocarcinoma. Gastrointest Endosc. 2011;74:51-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Goetz M, Malek NP, Kanz L, Hetzel J. Cryorecanalization for in-stent recanalization in the esophagus. Gastroenterology. 2014;146:1168-1170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Cash BD, Johnston LR, Johnston MH. Cryospray ablation (CSA) in the palliative treatment of squamous cell carcinoma of the esophagus. World J Surg Oncol. 2007;5:34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Greenwald BD, Dumot JA, Abrams JA, Lightdale CJ, David DS, Nishioka NS, Yachimski P, Johnston MH, Shaheen NJ, Zfass AM. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc. 2010;71:686-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Shah MB, Schnoll-Sussman F. Novel use of cryotherapy to control bleeding in advanced esophageal cancer. Endoscopy. 2010;42 Suppl 2:E46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Naidu H, Huang Q, Mashimo H. Gastric antral vascular ectasia: the evolution of therapeutic modalities. Endosc Int Open. 2014;2:E67-E73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Stotzer PO, Willén R, Kilander AF. Watermelon stomach: not only an antral disease. Gastrointest Endosc. 2002;55:897-900. [PubMed] [Cited in This Article: ] |
| 24. | Lecleire S, Ben-Soussan E, Antonietti M, Goria O, Riachi G, Lerebours E, Ducrotté P. Bleeding gastric vascular ectasia treated by argon plasma coagulation: a comparison between patients with and without cirrhosis. Gastrointest Endosc. 2008;67:219-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | McGorisk T, Krishnan K, Keefer L, Komanduri S. Radiofrequency ablation for refractory gastric antral vascular ectasia (with video). Gastrointest Endosc. 2013;78:584-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Dray X, Repici A, Gonzalez P, Kantsevoy SV, Fristrup C, Wengrower D, Camus M, Carlino A, Pérez-Roldán F, Adar T. Marteau PR. 1040 Radiofrequency Ablation Treatment of Gastric Antral Vascular Ectasia: Results From an International Collaborative Study. Gastrointest Endosc. 2013;77:AB180. [DOI] [Cited in This Article: ] |
| 27. | Kantsevoy SV, Cruz-Correa MR, Vaughn CA, Jagannath SB, Pasricha PJ, Kalloo AN. Endoscopic cryotherapy for the treatment of bleeding mucosal vascular lesions of the GI tract: a pilot study. Gastrointest Endosc. 2003;57:403-406. [PubMed] [Cited in This Article: ] |
| 28. | Cho S, Zanati S, Yong E, Cirocco M, Kandel G, Kortan P, May G, Marcon N. Endoscopic cryotherapy for the management of gastric antral vascular ectasia. Gastrointest Endosc. 2008;68:895-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Patel J, Parra V, Kedia P, Sharaiha RZ, Kahaleh M. Salvage cryotherapy in portal hypertensive gastropathy. Gastrointest Endosc. 2015;81:1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Talley NA, Chen F, King D, Jones M, Talley NJ. Short-chain fatty acids in the treatment of radiation proctitis: a randomized, double-blind, placebo-controlled, cross-over pilot trial. Dis Colon Rectum. 1997;40:1046-1050. [PubMed] [Cited in This Article: ] |
| 31. | Sebastian S, O’Connor H, O’Morain C, Buckley M. Argon plasma coagulation as first-line treatment for chronic radiation proctopathy. J Gastroenterol Hepatol. 2004;19:1169-1173. [PubMed] [Cited in This Article: ] |
| 32. | Zhou C, Adler DC, Becker L, Chen Y, Tsai TH, Figueiredo M, Schmitt JM, Fujimoto JG, Mashimo H. Effective treatment of chronic radiation proctitis using radiofrequency ablation. Therap Adv Gastroenterol. 2009;2:149-156. [PubMed] [Cited in This Article: ] |
| 33. | Hou JK, Abudayyeh S, Shaib Y. Treatment of chronic radiation proctitis with cryoablation. Gastrointest Endosc. 2011;73:383-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |