Published online Apr 16, 2013. doi: 10.4253/wjge.v5.i4.148
Revised: December 27, 2012
Accepted: January 23, 2013
Published online: April 16, 2013
Processing time: 215 Days and 2.1 Hours
AIM: To estimate the fetal radiation exposure using thermoluminescent dosimeters (TLD’s) in pregnant patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) and assess its relevance.
METHODS: Data on thirty-five therapeutic ERCPs conducted in pregnant patients from 2001 to 2009 were retrieved from a prospective database. Techniques to minimize fluoroscopy time were implemented and the fluoroscopy times captured. TLD’s were placed on the mother to estimate the fetal radiation exposure and the results were compared to the maximum allowed dose of radiation to the fetus [0.005 gray (Gy)]. Obstetrics consultations were obtained and the fetus was monitored before and after the ERCP. Fluoroscopy was performed at 75 kVp. ERCP was performed with the patients supine by dedicated biliary endoscopists performing more than 500 cases a year.
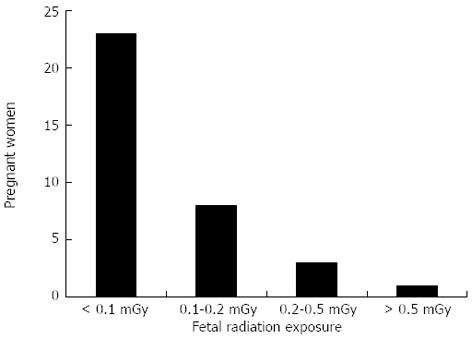
RESULTS: A total of 35 pregnant patients underwent ERCP and biliary sphincterotomy (14 in first trimester, 11 in second trimester, and 10 in third trimester). Mean maternal age was 25 years (range 16-37 years) and mean gestational age was 18.9 wk (range 4-35 wk). Mean fluoroscopy time was 0.15 min (range 0-1 min). For 23 women, the estimated fetal radiation exposure was almost negligible (< 0.0001 Gy) while for 8 women, it was within the 0.0001-0.0002 Gy range. Three women had an estimated fetal radiation exposure between 0.0002 and 0.0005 Gy and 1 woman had an estimated fetal radiation exposure greater than 0.0005 Gy. Complications included 2 post-sphincterotomy bleeds, 2 post-ERCP pancreatitis, and 1 fatal acute respiratory distress syndrome. One patient developed cholecystitis 2 d after ERCP.
CONCLUSION: ERCP with modified techniques is safe during pregnancy, and estimating the fetal radiation exposure from the fluoroscopy time or measuring it via TLD’s is unnecessary.
- Citation: Smith I, Gaidhane M, Goode A, Kahaleh M. Safety of endoscopic retrograde cholangiopancreatography in pregnancy: Fluoroscopy time and fetal exposure, does it matter? World J Gastrointest Endosc 2013; 5(4): 148-153
- URL: https://www.wjgnet.com/1948-5190/full/v5/i4/148.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i4.148
Choledocholithiasis can occur in as many as 12% of pregnant women and increases with gestational age[1]. It may be associated with cholangitis and/or gallstone pancreatitis, both of which have an increased morbidity for the mother and fetus[2]. Therefore choledocholithiasis is the most common indication for endoscopic retrograde cholangiopancreatography (ERCP) during pregnancy[3]. For pancreaticobiliary diseases in pregnancy, ERCP has been suggested as an effective alternative to surgery[4]. Suggestions have been made that ERCP is likely best performed during the second trimester, though the procedure appears reasonably safe to be performed throughout the entire period of pregnancy[5]. ERCP is currently exclusively indicated for therapeutic reasons in light of the endoscopic risks (such as bleeding, pancreatitis or perforation) as well as the ionizing radiation exposure to the fetus[6]. ERCPs are therapeutic when one or more of the following is performed: endoscopic sphincterotomy, removal of stones, stent placement, dilation of strictures. Efforts to minimize ionizing radiation, measured in rads (radiation absorbed dose)[5] or in rem (radiation equivalent man) or in international units gray (Gy)[7], should be undertaken. During neuron development, the threshold for malformations appears to be 0.001 Gy[5] and the overall maximum allowed dose of radiation to the fetus is 0.005 Gy[7]. The International Commission of Radiological Protections recommends specific calculations of fetal radiation exposure when doses are suspected to exceed the threshold of 0.01 Gy[8]. Our study sought to estimate the fetal radiation exposure using thermoluminescent dosimeters (TLD’s) in pregnant women undergoing therapeutic ERCP with modified techniques as well as look at the outcome of the ERCP in those patients.
All pregnant woman undergoing ERCP between 2001 till 2009 were captured in a dedicated prospective database. A total of thirty-five pregnant women were entered. The records were reviewed to determine the procedure indications and outcome in terms of success and eventual morbidity. Also, existing perinatal records were reviewed. The institutional review board approved the study protocol.
Pre-ERCP diagnosis included gallstone pancreatitis (17), choledocholithiasis (11), symptomatic cholelithiasis (6) and cholangitis (1). Obstetrics consultations were obtained and the fetus was monitored before and after the ERCP. Antibiotics were administered prophylactically. The modified technique involved the patients being placed supine on the fluoroscopy table, and the lower abdomen and pelvis being shielded with a 0.5- to 1.0-mm thickness of lead or its equivalent[7]. The uterus was positioned outside the primary X-ray beam. Four pairs of TLD’s were taped to the skin; one pair on the abdomen over the uterus shielded by lead, one pair on the upper abdomen in the primary beam, one pair on the lower back beneath the uterus shielded by lead and one pair on the upper back in the primary beam[7]. Fluoroscopy was performed at 75 kVp. A TLD reader was used and its readings were converted to milliards (mrads) of dose received at the skin surface by using a calibration curve[7]. TLDs on the upper back in the primary beam recorded the highest dose; about 10% of this dose was estimated to be the fetal dose[7]. The fetus was considered to be 10 cm from the posterior surface, and percentage depth dose at 10 cm was taken as approximately 10%. The depth dose varies with body habitus and gestational age and hence the dose estimation was an approximation[7].
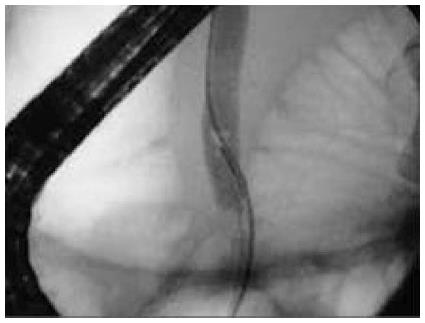
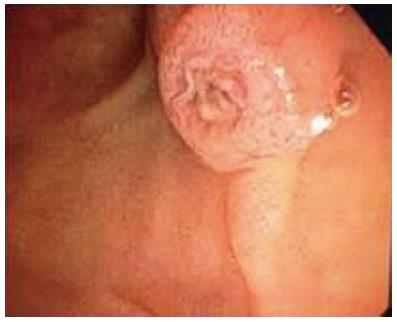
ERCP was performed with the patients supine by dedicated biliary endoscopists performing more than 500 cases a year[7]. Free biliary cannulation was obtained by using a sphincterotome and was confirmed by aspiration of bile, after which a biliary sphincterotomy was performed[7]. An 11.5-mm diameter retrieval balloon was advanced into the bile duct[7]. Contrast medium was injected, and a balloon occlusion cholangiogram was obtained to confirm the presence and location of stones, as well as cystic duct patency, after which the balloon was used to extract stones (Figures 1 and 2)[7].
A total of 35 pregnant patients underwent ERCP and biliary sphincterotomy (14 in first trimester, 11 in second trimester, and 10 in third trimester). Mean maternal age was 25 years (range 16-37 years) and mean gestational age was 18.9 wk (range 4-35 wk). Mean fluoroscopy time was 0.15 min (range 0-1 min). For 23 women, the estimated fetal radiation exposure was negligible (< 0.0001 Gy) while for 8 women, it was within the 0.0001-0.0002 Gy range. Three women had an estimated fetal radiation exposure between 0.0002 and 0.0005 Gy and one woman had an estimated fetal radiation exposure greater than 0.0005 Gy (Figure 3). Mean values for biochemical tests obtained before ERCP were the following: aspartate aminotransferase 179 IU/L (range: 25-310 IU/L); alanine aminotransferase 210 IU/L (27-561 IU/L); alkaline phosphatase 162 IU/L (44-394 IU/L); and total bilirubin 2.4 mg/dL (0.2-5 mg/dL). Four patients prior to pregnancy had cholecystectomy, one patient had a cholecystectomy during the pregnancy and prior to ERCP, and four patients required cholecystectomy post-ERCP during their pregnancy.
The patients’ final diagnosis was made based on ERCP findings, that is, extraction of stone or stone fragments after biliary sphincterotomy. Final diagnosis included the following: choledocholithiasis (18), gallstone pancreatitis (14), cholelithiasis, microlithiasis, and cholestasis. Complications of the ERCP procedure included post-sphincterotomy bleeding in two patients (controlled by hemoclip placement), post-ERCP pancreatitis (pancreatitis that developed within a week after ERCP) in two patients that necessitated one and two days of hospitalization, and acute respiratory distress syndrome in one patient who passed away as a result. One patient had cholecystitis requiring laparoscopic cholecystectomy 2 d post-ERCP. Two patients had contractions post-ERCP that resolved with hydration and terbutaline administration, respectively. Four mothers were at term and 2 mothers were preterm. Labor was induced in 2 mothers with non eventful delivery.
The incidence of gallstone disease during pregnancy has been estimated to be between 4.5% to 12%[1,3]. Choledocholithiasis may lead to potentially life-threatening cholangitis and/or gallstone pancreatitis. Given the necessity of treating cholangitis and gallstone pancreatitis during pregnancy[3] with therapeutic ERCP, an estimate of the radiation exposure to the fetus from an uncomplicated ERCP procedure should be known. Several published studies have investigated post-ERCP complications (preterm births, pancreatitis, sphincterotomy bleed) in pregnant women with a few capturing the mean time of fluoroscopy.
The mean fluoroscopy time was 14 s (range 1-48 s) and with use of TLDs the fetal radiation exposure was estimated to be 0.0004 Gy (range 0.0001-0.0018 Gy) in Kahaleh et al[7]. Despite there being a correlation between fluoroscopy time and radiation exposure, each fluoroscopy time corresponded with a wide range of radiation exposures. Complications included one post-sphincterotomy bleed and one post-ERCP pancreatitis. Two of the 17 women developed third-trimester preeclampsia, and labor was induced in both. Thirteen of the 15 patients who delivered were contacted and they confirmed that their child was in good health. Similar but limited complications were seen in Jamidar et al[9]. Twenty-three pregnant patients underwent a total of 29 ERCPs with one post-ERCP pancreatitis. Also, there was one spontaneous abortion (3 mo after ERCP) and one neonatal death; however, casual relationship to ERCP was not clear.
In Tang et al[10] in 2009, the largest retrospective study on ERCP in pregnant women, 68 ERCPs were performed on 65 pregnant women. The median fluoroscopy time was 1.45 min (range 0-7.2 min) and 11 patients (16%) had post-ERCP pancreatitis Term pregnancy was achieved in 53 patients (89.8%). Patients having ERCP in the first trimester had the lowest percentage of term pregnancy (73.3%) and the highest risk of preterm delivery (20.0%) and low-birth-weight newborns (21.4%). None of the 59 patients with long-term follow-up had spontaneous fetal loss, perinatal death, stillbirth, or fetal malformation.
Gupta et al[11] reported on one of the longest follow-up periods on fetal outcome after ERCP. Eighteen pregnant women underwent ERCP and sphincterotomy (4 in the first trimester, 6 in the second, and 8 in the third) in which the location of the cannula in the bile duct was confirmed using ultrasound guidance in 5 patients and bile aspiration in 2 patients. Indications included elective ERCP in 14 and symptomatic choledocholithiasis in 4. Complications included a post-sphincterotomy bleed and a mild post-ERCP pancreatitis in another, who also had preterm delivery. Eleven of 18 patients had healthy children without any developmental or congenital abnormalities 11-years post ERCP follow-up.
Tiwari et al[12] conducted a systematic review of 19 studies including 214 ERCPs in pregnant women and the procedure related complications included spontaneous abortion (0.9%), fetal distress (0.6%) and post procedure pancreatitis (4.6%). Preterm birth occurred in 4.6% with majority of the APGAR score greater than 8. Post-procedure pancreatitis risk factors include: young age, female sex, history of pancreatitis, sphincter of oddi dysfunction, difficult cannulation and precut sphincterotomy[6]. Thus, post-ERCP pancreatitis does not adversely affect pregnancy-related outcomes, as reported previously[10]. Cholecystectomy was performed in a few of the patients reviewed and most likely does not appear to lead to preterm delivery and low birth weight[10].
In a few studies, biliary stents were placed not only when residual stones or fragments were present, but also in an effort to limit total fluoroscopy time[10]. Farca et al[13] placed 10-French biliary stents without sphincterotomy in 10 patients, all of which had uncomplicated pregnancies and deliveries. Daas et al[4] in 2009 (17 ERCPs in 10 patients) placed plastic biliary stents when large (> 10 mm) biliary stones were encountered or when there was doubt regarding complete stone clearance. Fluoroscopy was used in 6 cases with mean exposure time of 8 s. Most of the 10 pregnant women in the study required repeat ERCPs with one woman receiving 3 subsequent ERCPs without fluoroscopy and had to return postpartum for a definitive stone extraction.
Barthel et al[14] performed biliary sphincterotomy in 3 patients with gallstone pancreatitis despite the absence of choledocholithiasis; one patient had post-ERCP pancreatitis and none had recurrent pancreatitis and all pregnancies had healthy outcome. Tang et al[10] showed that prophylactic sphincterotomy during ERCP can effectively reduce the risk of recurrent biliary pancreatitis during pregnancy. Therefore, ERCP with biliary sphincterotomy was performed in all 35 patients in our study.
Some have advocated eliminating radiation exposure by biliary cannulation with a sphincterotome, confirmation of access by bile aspiration[9] followed by sphincterotomy and stone extraction with a balloon catheter[5]. With this technique of using wire-guided cannulation techniques to achieve bile duct access without use of fluoroscopy[15], there is lack of ductal system definition and additional stones may be missed[5]. Importantly, aspiration of bile into the catheter does not necessarily confirm whether the CBD or the cystic duct has been cannulated[5]. Although it is important to minimize radiation exposure during ERCP, without fluoroscopy, residual stones or debris can be left in the CBD and might lead to recurrent cholangitis with more serious effects on both the fetus and mother[5].
In Sharma et al[16] in 2008, 11 pregnant women underwent biliary sphincterotomy and stenting without fluoroscopy and had definitive ERCP and stone clearance after pregnancy. One patient with large common bile duct stone required mechanical lithotripsy while another required surgery. Of note, the indication for the ERCP in the study was choledocholithiasis not cholangitis or gallstone pancreatitis which carry an increased mortality to the mother and fetus and likely necessitate definitive ERCP during the pregnancy. Further studies are required to prove that the clinical efficiency of nonradiating ERCP remains at the same level with conventional fluoroscopically guided ERCP[15]. Girotra et al[17] described an alternative management strategy to conventional ERCP in pregnant women with choledocholithiasis and cholangitis detected using EUS and choledochoscopy.
Fluoroscopy time can be utilized in ERCPs performed in pregnant patients and limiting fluoroscopy time is one of the most efficient methods to reduce radiation dose[3]. Lead shielding should be used[6] hard copy radiographs should be avoided[5] and anterior posterior beam projection should be used as it results in lower fetal dosing[6,8]. The radiation risks include fetal death, growth retardation especially during organogenesis and malformations[7]. Exposures over 0.001 Gy during neuron development and migration may be associated with microcephaly, mental retardation and childhood cancers[5]. The maximum allowed dose of radiation to the fetus is 0.005 Gy[7].
The International Commission of Radiological Protections recommends specific calculations of fetal radiation exposure when doses are suspected to exceed the threshold of 0.01 Gy[8]. Surprisingly, ERCP-induced fetal radiation exposure from ERCPs carried out in pregnant patients have been reported in the literature to vary from 0.0001 to 0.003 Gy per procedure[1,3,7,9,18,19]. In our study, the ERCP-induced fetal radiation ranged from less than 0.0001 to greater than 0.0005 Gy. For the majority of the women (88.6%), the estimated fetal radiation exposure was no more than 0.0002 Gy; while only one woman’s estimated fetal radiation exposure was greater than 0.0005 Gy. The fetal radiation exposure values in our study are below the threshold established by the International Commission of Radiological Protections needing specific calculations of fetal radiation exposure and the maximum allowed dose of radiation to the fetus.
Thus, for a routine ERCP with modified techniques, estimating the fetal radiation exposure from the fluoroscopy time and measuring it with the use of TLD’s is unnecessary. The threshold may be exceeded in complicated long-lasting ERCPs[3] and in these complicated long-lasting ERCPs, dosimetry may be used to estimate the fetal radiation exposure, such as patients with altered anatomy, failed prior ERCP or complex bile leak. By placing TLD’s on the pregnant patient over and above the uterus, one can obtain a good estimate of the fetus doses from calculations based on a TLD reading. The value is an approximation, probably an underestimate of the real value, as the principal source of radiation to the fetus during the ERCP comes from scattered radiation absorbed within the mother’s body[3]. Tham et al[1] attempted to attain a better estimate using nonanthopomorphic phantom to estimate the entrance skin dose and estimated the fetal dose exposure at 0.003 Gy.
The safety and efficacy of therapeutic ERCP has been demonstrated in many studies[1,7,9,11,13,20-31]. For a routine ERCP, the reported fetal radiation exposure falls below the maximum allowed dose of radiation to the fetus of 0.005 Gy[7], therefore estimating the fetal radiation exposure from the fluoroscopy time or by measuring it from the use of TLD’s is unnecessary.
For pancreaticobiliary diseases in pregnancy, endoscopic retrograde cholangiopancreatography (ERCP) has been suggested as an effective alternative to surgery. ERCPs are therapeutic when one or more of the following is performed: endoscopic sphincterotomy, removal of stones, stent placement, dilation of strictures.
Fluoroscopy time can be utilized in ERCPs performed in pregnant patients and limiting fluoroscopy time is one of the most efficient methods to reduce radiation dose.
The fetal radiation exposure values in the authors’ study are below the threshold established by the International Commission of Radiological Protections needing specific calculations of fetal radiation exposure and the maximum allowed dose of radiation to the fetus.
The aim of the present article is the estimation of the fetal radiation exposure using TLD’s in pregnant women undergoing ERCPs. The article is sound and deserves publication.
P- Reviewers Arcidiacono PG, Sudhoff H S- Editor Zhai HH L- Editor A E- Editor Zhang DN
| 1. | Tham TC, Vandervoort J, Wong RC, Montes H, Roston AD, Slivka A, Ferrari AP, Lichtenstein DR, Van Dam J, Nawfel RD. Safety of ERCP during pregnancy. Am J Gastroenterol. 2003;98:308-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Scott LD. Gallstone disease and pancreatitis in pregnancy. Gastroenterol Clin North Am. 1992;21:803-815. [PubMed] [Cited in This Article: ] |
| 3. | Samara ET, Stratakis J, Enele Melono JM, Mouzas IA, Perisinakis K, Damilakis J. Therapeutic ERCP and pregnancy: is the radiation risk for the conceptus trivial? Gastrointest Endosc. 2009;69:824-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Daas AY, Agha A, Pinkas H, Mamel J, Brady PG. ERCP in pregnancy: is it safe? Gastroenterol Hepatol (N Y). 2009;5:851-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Al-Hashem H, Muralidharan V, Cohen H, Jamidar PA. Biliary disease in pregnancy with an emphasis on the role of ERCP. J Clin Gastroenterol. 2009;43:58-62. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Baron TH, Schueler BA. Pregnancy and radiation exposure during therapeutic ERCP: time to put the baby to bed? Gastrointest Endosc. 2009;69:832-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Kahaleh M, Hartwell GD, Arseneau KO, Pajewski TN, Mullick T, Isin G, Agarwal S, Yeaton P. Safety and efficacy of ERCP in pregnancy. Gastrointest Endosc. 2004;60:287-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | International Commission on Radiological Protection. Pregnancy and medical radiation. Ann ICRP. 2000;30:iii-viii, 1-43. [PubMed] [Cited in This Article: ] |
| 9. | Jamidar PA, Beck GJ, Hoffman BJ, Lehman GA, Hawes RH, Agrawal RM, Ashok PS, Ravi TJ, Cunningham JT, Troiano F. Endoscopic retrograde cholangiopancreatography in pregnancy. Am J Gastroenterol. 1995;90:1263-1267. [PubMed] [Cited in This Article: ] |
| 10. | Tang SJ, Mayo MJ, Rodriguez-Frias E, Armstrong L, Tang L, Sreenarasimhaiah J, Lara LF, Rockey DC. Safety and utility of ERCP during pregnancy. Gastrointest Endosc. 2009;69:453-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Gupta R, Tandan M, Lakhtakia S, Santosh D, Rao GV, Reddy DN. Safety of therapeutic ERCP in pregnancy - an Indian experience. Indian J Gastroenterol. 2005;24:161-163. [PubMed] [Cited in This Article: ] |
| 12. | Tiwari P, Khan AS, Nass JP, Rivera RE, Romero RV, Antillon MR, Roy PK. Mo1578 ERCP in Pregnancy: A Systematic Review. Gastrointest Endosc. 2011;73:AB392-AB393. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Farca A, Aguilar ME, Rodriguez G, de la Mora G, Arango L. Biliary stents as temporary treatment for choledocholithiasis in pregnant patients. Gastrointest Endosc. 1997;46:99-101. [PubMed] [Cited in This Article: ] |
| 14. | Barthel JS, Chowdhury T, Miedema BW. Endoscopic sphincterotomy for the treatment of gallstone pancreatitis during pregnancy. Surg Endosc. 1998;12:394-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Shelton J, Linder JD, Rivera-Alsina ME, Tarnasky PR. Commitment, confirmation, and clearance: new techniques for nonradiation ERCP during pregnancy (with videos). Gastrointest Endosc. 2008;67:364-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Sharma SS, Maharshi S. Two stage endoscopic approach for management of choledocholithiasis during pregnancy. J Gastrointestin Liver Dis. 2008;17:183-185. [PubMed] [Cited in This Article: ] |
| 17. | Girotra M, Jani N. Role of endoscopic ultrasound/SpyScope in diagnosis and treatment of choledocholithiasis in pregnancy. World J Gastroenterol. 2010;16:3601-3602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Axelrad AM, Fleischer DE, Strack LL, Benjamin SB, al-Kawas FH. Performance of ERCP for symptomatic choledocholithiasis during pregnancy: techniques to increase safety and improve patient management. Am J Gastroenterol. 1994;89:109-112. [PubMed] [Cited in This Article: ] |
| 19. | Howden JK, Robuck-Mangum G, Jowell PS, Branch MS, Yoshizumi T, Swartz KL, Baillie J. Endoscopic management of symptomatic choledocholithiasis (CDL) during pregnancy: Safety and efficacy of endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic sphincterotomy (ES). Gastrointest Endosc. 2001;53:AB96. [DOI] [Cited in This Article: ] |
| 20. | Baillie J, Cairns SR, Putman WS, Cotton PB. Endoscopic management of choledocholithiasis during pregnancy. Surg Gynecol Obstet. 1990;171:1-4. [PubMed] [Cited in This Article: ] |
| 21. | Menees S, Elta G. Endoscopic retrograde cholangiopancreatography during pregnancy. Gastrointest Endosc Clin N Am. 2006;16:41-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Cappell MS. The fetal safety and clinical efficacy of gastrointestinal endoscopy during pregnancy. Gastroenterol Clin North Am. 2003;32:123-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Swisher SG, Hunt KK, Schmit PJ, Hiyama DT, Bennion RS, Thompson JE. Management of pancreatitis complicating pregnancy. Am Surg. 1994;60:759-762. [PubMed] [Cited in This Article: ] |
| 24. | Qureshi WA, Rajan E, Adler DG, Davila RE, Hirota WK, Jacobson BC, Leighton JA, Zuckerman MJ, Hambrick RD, Fanelli RD. ASGE Guideline: Guidelines for endoscopy in pregnant and lactating women. Gastrointest Endosc. 2005;61:357-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Tarnasky PR, Simmons DC, Schwartz AG, Macurak RB, Edman CD. Safe delivery of bile duct stones during pregnancy. Am J Gastroenterol. 2003;98:2100-2101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 26. | Simmons DC, Tarnasky PR, Rivera-Alsina ME, Lopez JF, Edman CD. Endoscopic retrograde cholangiopancreatography (ERCP) in pregnancy without the use of radiation. Am J Obstet Gynecol. 2004;190:1467-1469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Sungler P, Heinerman PM, Steiner H, Waclawiczek HW, Holzinger J, Mayer F, Heuberger A, Boeckl O. Laparoscopic cholecystectomy and interventional endoscopy for gallstone complications during pregnancy. Surg Endosc. 2000;14:267-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Howden JK, Baillie J. Preoperative versus postoperative endoscopic retrograde cholangiopancreatography in mild to moderate pancreatitis: a prospective randomized trial. Gastrointest Endosc. 2001;53:834-836. [PubMed] [Cited in This Article: ] |
| 29. | Akcakaya A, Ozkan OV, Okan I, Kocaman O, Sahin M. Endoscopic retrograde cholangiopancreatography during pregnancy without radiation. World J Gastroenterol. 2009;15:3649-3652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Chong VH, Jalihal A. Endoscopic management of biliary disorders during pregnancy. Hepatobiliary Pancreat Dis Int. 2010;9:180-185. [PubMed] [Cited in This Article: ] |
| 31. | García-Cano J, Pérez-Miranda M, Pérez-Roldán F, González-Carro P, González-Huix F, Rodríguez-Ramos C, Naranjo A, González-Martín JÁ, de la Serna C. ERCP during pregnancy. Rev Esp Enferm Dig. 2012;104:53-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |