Published online Nov 16, 2011. doi: 10.4253/wjge.v3.i11.201
Revised: September 5, 2011
Accepted: October 20, 2011
Published online: November 16, 2011
Obesity is a serious health problem in the United States. Although laparoscopic surgical procedures are effective in achieving weight loss and improving obesity-related co-morbidities, they are not without their limitations and consequently there is a growing demand for less invasive approaches. Transoral techniques, as both primary and revisional procedures, are promising in this regard as they may provide a safer and more cost-effective means of achieving meaningful weight loss. The aim of this paper is to review the currently available transoral approaches to weight loss, with a particular focus on those applied in human trials.
- Citation: Noria SF, Mikami DJ. Transoral surgery for morbid obesity. World J Gastrointest Endosc 2011; 3(11): 201-208
- URL: https://www.wjgnet.com/1948-5190/full/v3/i11/201.htm
- DOI: https://dx.doi.org/10.4253/wjge.v3.i11.201
Obesity is a serious public health problem associated with increased morbidity and mortality and decreased quality of life. According to the World Health Organization, in 2005 there were approximately 1.6 billion overweight adults and at least 400 million obese adults worldwide[1]. The prevalence of obesity has increased so rapidly over the last few decades that it is now considered a global epidemic.
In the United States, the National Health and Nutrition Examination Surveys, conducted by the Center for Disease Control, study the prevalence of obesity by using directly measured heights and weights. Studies have shown that currently there are 72 million obese adults [i.e., body mass index (BMI) ≥ 30 kg/m2]. Interestingly, while the prevalence has more than doubled over the last four decades (from 13.4% in 1960-1962 to 35.1% in 2005-2006 for adults aged 20-74 years)[2], it seems to have reached a plateau over the last 3 years[3-5]. However, when Ogden et al[4] compared the distribution of BMI between 1976-1980 and 2005-2006, they observed that, among adults, the distribution of BMI shifted to the right, reflecting the change in prevalence of super obesity (i.e., BMI ≥ 50 kg/m2), which increased from 0.9% in 1960-1962 to 6.2% in 2005-2006 among adults.
Studies have indicated that obesity is responsible for more than 2.5 million deaths worldwide per year[6] due to the increased prevalence of related co-morbidities, including type 2 diabetes, hyperlipidemia, hypertension, obstructive sleep apnea, heart disease, stroke, asthma, back and lower extremity weight-bearing degenerative problems, several forms of cancer and depression[6-8]. Additionally, obesity is an independent risk factor for mortality. A study by Fontaine et al[9] demonstrated that, in comparison with a normal weight individual, a 25-year-old morbidly obese man has a 22% reduction in life expectancy, representing approximately 12 years of life lost. A more recent study examining 10-year mortality rates in more than 500 000 Americans, 50 to 71 years old, demonstrated that in middle aged men and women who were non-smokers and had no pre-existing illnesses, there was a 20%-40% increased mortality in those who were overweight (i.e., BMI 25-30 kg/m2) and a 2- to 3-fold increased risk of mortality in individuals who were obese (BMI ≥ 30 kg/m2)[10].
As is evidenced by the innumerable weight loss programs, most adults attempt to lose weight at some point in their life[11]. However, medically managed weight loss, including diets and pharmaceutical agents, are ineffective in the long-term treatment of obesity[12]. In 1991, the National Institutes of Health established guidelines for the surgical management of morbid obesity (BMI ≥ 40 kg/m2 or BMI > 35 kg/m2 in the presence of significant co-morbidities)[13,14] and, since then, the number of bariatric surgical procedures has dramatically increased. In 2004, approximately 144 000 obese individuals received surgical treatment, compared to the 20 000 procedures performed in 1999[15]. The dramatic increase is most likely the result of refinement in minimally invasive surgical techniques, increased media coverage and increased patient satisfaction. Indeed, of the various available weight-loss strategies, bariatric surgery is the only effective long-term weight-loss therapy for obese patients[16].
Bariatric surgical procedures are divided into restrictive (i.e., adjustable gastric banding, vertical banded gastroplasty, sleeve gastrectomy), malabsorptive (biliopancreatic diversion with/out duodenal switch) or a combination of both (roux-en-y gastric bypass). Of the various procedures, roux-en-y gastric bypass and adjustable gastric banding are the most commonly performed procedures. While bariatric surgery has been shown to be extremely effective for long-term weight loss, the mortality rate, albeit low, is not zero [i.e., 0.28% (95% CI: 0.22-0.34) and 0.35% (95% CI: 0.12-0.58) at ≤ 30 d and > 30 d respectively[17]]. Additionally, from a morbidity perspective, there are procedure-specific risks[18,19] and shared complications, including incisional hernias, wound infections, fistula and leaks[20].
More recently, there has been emerging interest in transoral techniques for pre-operative, stand-alone or revisional bariatric procedures[21]. Specifically, considering transoral surgery is performed exclusively through the gastrointestinal (GI) tract via a flexible endoscope, the value of this approach lies in the possibility of an ambulatory weight loss procedure that may be safer and more cost effective compared with laparoscopic approaches. By extension, this may allow bariatric procedures to be performed in those individuals who are currently precluded due to multiple co-morbidities, older age, super-obesity (BMI ≥ 50 kg/m2), mild obesity (BMI 25-30 kg/m2), atypical anatomy (e.g., adhesions secondary to previous abdominal surgery, a history of gastric resection, or bowel resection) or disease states that affect the bowel (e.g., Crohn’s disease). The present paper aims to review currently available transoral techniques with a focus on those applied in human trials.
Primary procedures are divided into restrictive or malabsorptive. Restrictive devices include intragastric balloons, endolumenal suturing, endolumenal stapling and the transoral restrictive implant system. These are designed to mimic restrictive laparoscopic procedures (i.e., adjustable gastric banding, vertical banded gastroplasty, sleeve gastrectomy). Malabsorptive procedures (i.e., biliopancreatic diversion with/out a duodenal switch) induce weight loss by bypassing the absorptive surface of the intestine. The duodenal-jejunal bypass sleeve is a malabsorptive device that mimics such surgical procedures.
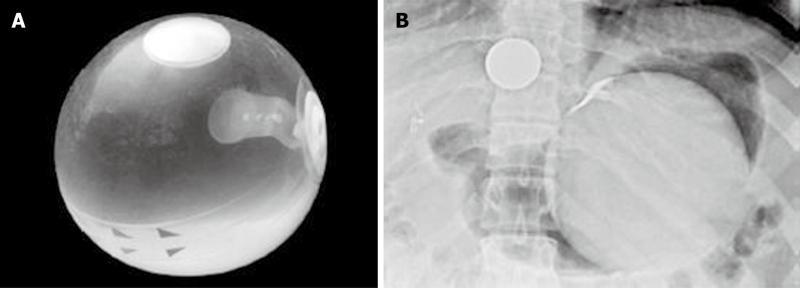
One of the earliest transoral devices created to restrict oral intake was the intragastric balloon. Unfortunately, significant complications, premature balloon deflation and failure to achieve meaningful weight loss led to it being abandoned as a weight loss device. However, in 1987, guidelines for development of an appropriate balloon appliance were outlined and the BioEnterics Intragastric Balloon (BIB; Inamed, Santa Barbara CA) was developed[22]. Structurally, the BIB is a small, flexible balloon in the collapsed state and expands into a spherical shape 10 cm in diameter when filled with 500 mL of saline solution. Its shell is made of an inert, nontoxic silicone elastomer that is resistant to gastric acid. The balloon has a radiopaque self-sealing valve that allows volume adjustments from 400 to 800 mL.
Procedurally, under conscious sedation or general anesthesia, a diagnostic endoscopy should be performed to rule out abnormalities that would preclude balloon insertion. The BIB placement assembly, consisting of a sheath with the collapsed balloon and a balloon fill tube, is then inserted into the gastric fundus. A syringe, attached to the balloon fill tube, is then used to fill the balloon under direct visualization with 500-700 mL of saline/methylene blue solution. After filling the balloon, gentle suction exerted by withdrawing the plunger of the syringe creates a vacuum, which seals the valve. The balloon is released by a short pull on the fill tube and this tube with the placement assembly is removed. After placement, the position of the free-floating balloon can be confirmed radiographically (Figure 1)[22]. BIB adjustments, to increase or decrease volume if there is inadequate weight loss or persistent nausea and vomiting respectively, requires endoscopy and a reintubation catheter. Directional arrows at the equator of the balloon assist in identifying the valve and the reintubation catheter is pushed into the valve to add or remove fluid. Finally, to remove the BIB, as much fluid as possible is removed before grasping the balloon with a snare or forceps. The endoscope and the grasped balloon are then gently removed. A needle is available to puncture the balloon in case of unsuccessful reintubation. In terms of morbidity, the major adverse events include nausea and vomiting that in some cases can require early removal, as well as early deflation, gastric ulceration and erosion[22].
Genco et al[23] conducted a retrospective analysis to assess the efficacy of the BIB in terms of weight loss and improvement in obesity-related co-morbid states. From 2002-2004, 2515 patients with a mean BMI of (44.8 ± 7.8) kg/m2 underwent endoscopic placement of the BIB. Positioning of the balloon was successful in all but 2 cases (0.08%), and the overall complication rate was 2.8% (70/2515). Specifically, gastric perforation occurred in 5 patients, 4 of whom had undergone previous gastric surgery, and 2 of whom died. There were 19 gastric obstructions (0.76%) within the first week of insertion and these were remedied by BIB removal. The balloon ruptured in 9 cases (0.36%) and had to be removed. Finally, esophagitis was diagnosed in 32 patients (1.27%) and gastric ulcers developed in 5 patients (0.2%) with a history of peptic disease. Both complications were treated with medical therapy.
Concerning weight loss and improvement in obesity-related co-morbidities, at 6 mo follow-up, the percentage excess weight loss (%EWL) was (33.9 ± 18.7). During this interval, improvement or resolution of diabetes and hypertension was observed in 86.9% and 93.7%, respectively. These results were further supported in a randomized, sham-controlled, crossover study of 32 patients conducted by the same group[24].
Taken together, studies demonstrate that the BIB, in conjunction with the appropriate diet, is a safe, effective short-term weight loss procedure in patients that have not had any previous gastric surgery. As such, its role should be relegated to being a bridge to more definitive bariatric interventions. Currently, however, no intragastric balloons are approved for use in the United States.
Endoluminal vertical gastroplasty (EVG), using the Bard EndoCinch Suturing System (C.R. Bard, Murray Hill, NJ), was first described in the context of treating gastroesophageal reflux disease. Due to lack of repair durability, the role of EVG in control of gastroesophageal reflux disease (GERD) was abandoned[25-27].
In terms of its application for the treatment of obesity, Fogel et al[28] first described the use of the EndoCinch in 64 patients. The primary objectives of this study were to determine the safety and technical feasibility of EVG. The secondary aim was assessment of its efficacy with respect to patient weight loss. Technically, the EVG involves configuring one continuous suture running through 5 to 7 stitch points, in a cross-linked fashion from proximal fundus to distal body[28]. The suture is deployed from a capsule attached to the end of a diagnostic gastroscope. Specifically, as described by Fogel et al[28], the first stitch is placed proximally in the nearest folds on the anterior face of the gastric fundus (approximately 40-43 cm from the mouth). Subsequently, a second stitch is placed as far down on the anterior face to the most distal fold of the stomach body’s rugae, proximal to the antrum, usually 10 to 13 cm from the first stitch (approximately 53 cm from the mouth). A third stitch is then placed 1 to 2 cm proximal to the second stitch but on the posterior face of the stomach (approximately 51 cm from the mouth). Subsequent stitches are placed, working in a proximal direction alternating anterior and posterior faces of the stomach with consecutive stitches separated by approximately 2 cm. The last stitch in the sequence is placed on the posterior face 1 to 2 cm proximal from the first stitch. After all the stitches are placed and visualized, the suture is tightened, bringing the anterior and posterior faces together creating the EVG. The suture is then secured and excess suture is cut.
In terms of outcomes, Fogel et al[28] demonstrated that the mean procedure time was 45 min with a recovery time of 1 to 2 h. All patients were discharged on the day of procedure. No patients experienced any serious adverse events. However, minor events included nausea and reflux-like symptoms that resolved within 24 h. Follow-up for up to 12 mo was accomplished in 59 of 64 patients. Results for secondary outcomes demonstrated a significant reduction in BMI at 12 mo [mean ± SD, BMI (39.9 ± 5.1) kg/m2 at baseline vs (30.6 ± 4.7) kg/m2 at 12 mo; P < 0.001] with mean (%EWL ± SD) of (21.1 ± 6.2), (39.6 ± 11.3), and (58.1 ± 19.9) at 1, 3 and 12 mo post-procedure, respectively. Additionally, repeat endoscopy performed in a non-uniform manner in 14 of 64 patients after reports of feeling hungry or plateauing weight loss, demonstrated 11 patients had intact suture lines but three had disrupted sutures. These were repaired by repeating the suturing procedure.
A newer version of the EVG device was created and tested by Brethauer et al[29] in 18 patients in a short term (≤ 24 h) study. They demonstrated that the average procedure time was (125 ± 23) min and there were no serious procedure related complications. Minor complaints included nausea, vomiting and abdominal discomfort.
Issues that still need to be addressed include the long-term durability of the plication procedure given the problems seen with earlier trials focused on the treatment of GERD. Additionally, the ease of repeating interventions to facilitate additional weight loss in refractory or recurrent cases needs to be examined.
The Transoral Gastroplasty System (TOGA; Satiety Inc., Palo Alto, CA) was the first endoscopic device to use staplers to create full thickness plications along the lesser curve of the stomach, effectively creating a sleeve[30]. The technique entails upper endoscopy and placement of a guide wire over which a 60 Fr bougie is introduced to dilate and test for any resistance prior to device introduction. The TOGA Sleeve Stapler is introduced over the guide wire and the wire removed. A ≤ 8.6 mm endoscope is introduced through a channel in the device, advanced into the stomach and retroflexed for direct viewing of the stapling procedure. In the stomach, the body of the stapler is positioned along the lesser curvature and the jaws opened. A septum with an attached retraction wire is deployed to spread and orient the stomach tissue for capture and stapling. Suction is applied and tissue from the anterior and posterior walls of the stomach is acquired into two vacuum pods in the device. The stapler is closed and fired, delivering three rows of 11 titanium staples. This creates a transmural staple line connecting the anterior and posterior stomach, beginning 1 cm proximal to the Z-line and extending distally 4.5 cm, parallel to the lesser curvature. This process is repeated to add a second staple line, extending the new sleeve distally to create a sleeve approximately 8-9 cm in length. The distal sleeve outlet is then narrowed using the TOGA Restrictor, a single-suction-pod stapler that acquires and staples tissue in pleats. Restrictions are placed until the outlet is less than 20 mm.
Devière et al[30] conducted the first human prospective, single-arm trial examining the safety and feasibility of the TOGA system in 21 patients. Primary outcomes focused on safety and appearance of the pouch at 6 mo. Secondary outcomes included %EWL. The study demonstrated that the most common device-related adverse events were vomiting, pain, nausea and transient dysphagia. There were no serious adverse events. Assessment of pouch anatomy at 6 mo demonstrated staple line gaps in 13 patients, incomplete distal sleeves in 3 patients and normal pouch anatomy (i.e., intact sleeves and staple lines) in 5 patients. In terms of secondary outcomes, mean EWL was 16.2%, 22.6%, and 24.4% at 1, 3, and 6 mo and the average BMI decreased from 43.0 pretreatment to 37.8 at 6 mo (P < 0.0001).
A follow-up prospective, single-arm study by the same group[31] examined the safety and feasibility of a second generation TOGA stapler that was modified to remedy the staple line gaps. The device was improved by the development of an adjustable septum that allowed closer apposition of the two staple lines. A total of 11 patients were recruited into the trial. No serious adverse events were observed but procedure related problems included transient epigastric pain requiring analgesic treatment, throat pain, esophagitis, nausea and mild dysphagia. At 6 mo, endoscopic examination demonstrated that 4 of 11 patients had a mid-stoma (less than 1cm) indicative of a gap between the first and second staple line. Assessment of %EWL demonstrated a reduction of 19.2 %, 33.7% and 46.0% at 1, 3 and 6 mo, respectively (P < 0.05), with a mean BMI reduction from 41.6 kg/m2 before treatment to 38.1 kg/m2, 35.4 kg/m2 and 33.1 kg/m2 at 1, 3 and 6 mo, respectively (P < 0.01).
Taken together, these studies demonstrate that the TOGA system is feasible, safe and can induce significant weight loss in the short-term. Currently the Phase I trials are being validated in multicenter, randomized controlled trials to evaluate the durability and extent of weight loss.
The transoral endoscopic restrictive implant system [BaroSense Trans-oral Endoscopic Restrictive Implant System (TERIS), BaroSense, Redwood City, CA] endoscopically implants a prosthetic device at the level of the cardia, creating a small gastric reservoir. The procedure requires formation of 5 gastric plications with insertion of 5 silicone anchors, followed by attachment of the gastric restrictor[32].
Specifically, plications are created at the level of the cardia, 3 cm distal to the gastroesophageal junction. The first plication is created just above the lesser curve of the stomach using an articulating endoscopic circular stapler which, through suction, can acquire a full-thickness gastric plication, compress the tissues and create 2 concentric rings of 3.5 mm staples reinforced by a plastic ring. The stapler also excises the tissues within the ring to create a plication hole. Then using a 2-lumen cannulation guide, the endoscope, silicone anchors and articulated guide with an anchor grasper are inserted. The proximal end of the silicone anchor is pulled under direct visualization through the plication hole and then released. Once all five anchors are placed, they are each attached to locking anchor graspers using a multiple-lumen guide and a 5mm endoscope. The 5 proximal handles of the anchor graspers are passed through the 5 apertures in the gastric restrictor and are used to guide the gastric restrictor down to the level of the anchors. Under direct visualization, the proximal ends of the silicone anchors are brought inside the gastric restrictor, to lock it in place.
The initial feasibility and safety of the TERIS system is being examined in 20 human subjects by Biertho et al[32] using a randomized, uncontrolled, open label, single group Phase I human trial. A published report on their first case demonstrated that there were no intra- or post-operative complications and the patient was discharged home on post-operative day 2 tolerating a soft diet. At 3 and 6 mo, the %EWL was 21% and 26% respectively.
Considering the novelty of the TERIS system, further investigation of the safety and efficacy of the device in both the short and long-term and comparison to controls in a randomized fashion is warranted.
The duodenal-jejunal bypass sleeve (DJBS, The EndoBarrier, GI Dynamics Inc., Lexington, MA, United States) is an endoluminal malabsorptive procedure that effectively bypasses the proximal small intestine using a 60 cm long fluoropolymer liner anchored in the duodenum. Under general anesthesia, the device is delivered using both fluoroscopy and endoscopy. The implant is delivered using an over-the-wire catheter system and is contained within a capsule at the distal end of the catheter. Once the capsule is placed in the duodenum, an inner catheter is pushed and the bowel negotiated with the aid of an atraumatic ball attached to the distal end of the catheter. The sleeve, which is attached to the catheter, is pulled out of the capsule as the catheter is advanced. Once the sleeve is fully deployed, the anchor is deployed from the capsule to sit within the duodenal bulb. The anchor is self-expanding and the barbs engage the tissue to prevent movement. Contrast is flushed to ensure patency of the sleeve and the sleeve and ball are detached from the catheter which is removed from the bowel, leaving the implant in place[33].
Rodriguez-Grunert et al[33] reported on the first human experience, delivering and retrieving the DJBS in 12 patients. Primary outcomes measured the incidence and severity of adverse events, with secondary measures focused on %EWL and changes in co-morbid status. The mean implant and explant times were 26.6 and 43.3 min respectively. The device remained in place for 12 wk in 10 of 12 patients, with early retrieval (i.e., 9 d) in 2 patients due to intractable abdominal pain. Most adverse events related to implantation occurred within the first 2 wk and included abdominal pain, nausea and vomiting. During explantation, there was one partial pharyngeal tear and one esophageal tear. All patients had implant site inflammation. In terms of weight loss, at 12 wk, the average %EWL in 10 of 12 patients was 23.6%, with all patients achieving at least a 10% EWL. Finally, of the 4 diabetic patients, all had normal fasting plasma glucose levels for the entire 12 wk without the need for oral hypoglycemics and 3 of 4 patients had decreased HbA1c of ≥ 0.5% by week 12.
Tarnoff et al[34] conducted an open-label, multicenter, prospective randomized control trial comparing the effect of the DJBS with a low fat diet, to a low fat diet alone for 12 wk. The device was implanted in 25 patients and 14 patients comprised the control arm. Both groups received counseling at baseline, consisting of a low calorie diet, with advice on exercise and behavior modification. The study demonstrated that 20 of 25 device subjects maintained the sleeve for 12 wk. The mean EWL was 22% and 5% for the device and control groups, respectively. Five of 25 device subjects had to have the device explanted early due to upper GI bleeding (n = 3), anchor migration (n = 1) and sleeve obstruction (n = 1). At 12 wk, the average %EWL was 22.1% and 5.3% for the device and control group respectively. Concerning diabetes, four patients (i.e. 1 control subject and 3 experimental subjects) had a history of type 2 diabetes. Within 1 wk, all 4 patients had improved HbA1c levels and one diabetic in the device arm had complete resolution of diabetes at 12 wk.
While future studies are needed to elucidate the safety and feasibility of the DJBS in both the short- and long-term, this procedure could potentially be utilized as a non-surgical method for pre-operative weight loss or improvement and/or resolution of type 2 diabetes. Additionally, the DJBS could be utilized as a bridge in those patients whose BMI and/or comorbidities preclude them from undergoing surgery, allowing weight reduction and improvement in co-morbidities to a level that would make a surgical bariatric procedure more safe.
Transoral procedures are also being investigated in the context of revisional bariatric surgery. Specifically, while roux-en-y gastric bypass remains the gold-standard surgical procedure for weight loss (i.e., %EWL at 2 years of 61.6%[16]; early and late mortality of 0.16% and 0.09%, respectively[17]), inadequate loss and/or weight regain is reported as high as 25%-30% after gastric bypass or other bariatric procedures[35,36]. The etiology of weight regain is multifactorial[37] and includes inadequate long-term management of psychological, dietary or medical issues, as well as anatomical aberrancies.
Focusing on anatomy, initial investigations must include an esophagogastroduodenoscopy (EGD) or upper GI study to evaluate for gastro-gastric fistula, gastric pouch dilatation or anastomotic dilatation. Once a gastro-gastric fistula is ruled out, gastrojejunal anastomosis and/or pouch dilatation may underlie weight regain as patients may lose the feeling of early satiety, leading to overeating. Indeed, upper endoscopy has revealed that, in patients who regain weight, the size of the stoma or anastomosis is twice the immediate post-operative diameter of 1.0-1.5 cm.
If dilatation of the pouch or gastrojejunal anastomosis is diagnosed, then revisional surgery may be necessary and issues related to feasibility and safety arise. Recent studies estimate a rate of 5%-13% for major complications with re-operative surgery[38], the most serious of which include anastomotic leaks, wound dehiscence, incisional hernias and pulmonary complications.
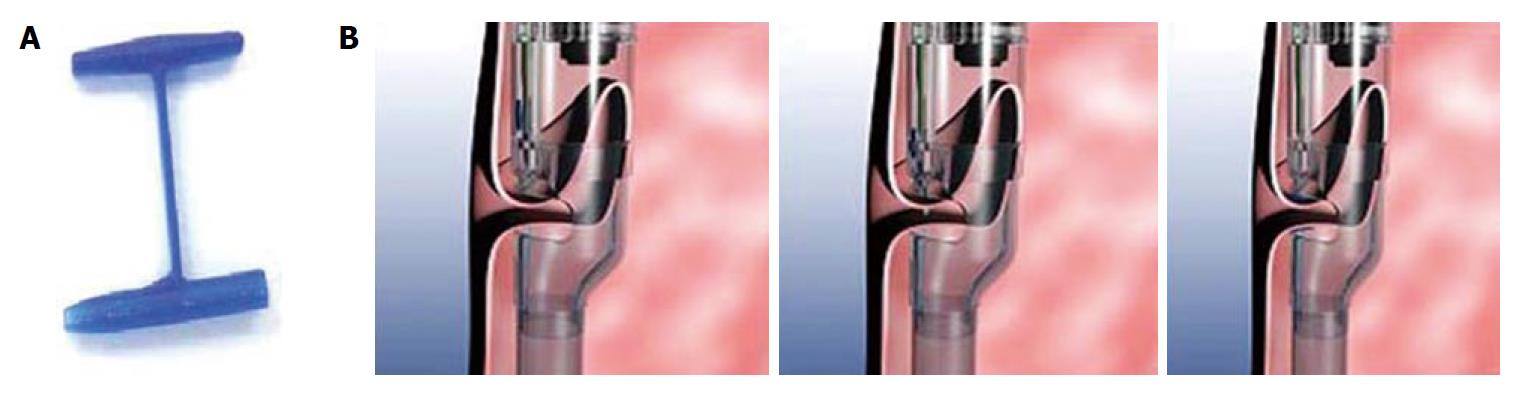
Given the frequency of weight regain and risk of revisional surgery, Mikami et al[39] investigated whether it was possible to restore a dilated gastric pouch to its original inner diameter and/or volume transorally by utilizing the StomaphyX™ device; a natural orifice surgical device that utilizes 7 mm, 3-0 polypropylene H-fasteners to create full-thickness, serosal-to-serosal tissue approximation.
As described by Mikami et al[39], under general anesthesia, an EGD is performed to measure the length of the pouch. The gastroscope is then placed through the internal lumen of the StomaphyX™ device and extended approximately 20 cm beyond the device. The apparatus is then passed down the esophagus, through the gastric pouch and the scope is passed into the efferent jejunal limb to allow passage of the StomaphyX™ device through the anastomosis. Using suction, the StomaphyX™
device draws tissue through an opening at its distal end and an H-fastener is deployed. In this manner, a circular pleat of tissue is created 1 cm proximal to the anastomosis, with a second row placed 1 cm proximal to the first row. A total of 12 fasteners are placed at two different levels. Finally, any identified open mucosal areas are fastened (i.e., an additional 3-5 fasteners). Repeat endoscopy is used at the end of the procedure to assess the reduction of the gastric pouch and anastomosis (Figure 2).
A total of 39 patients were enrolled in the trial[39]. The average procedure time was 35 min (16-62 min) and between 12 and 41 H-fasteners were used in each case. In terms of primary safety outcomes, 37 of 39 patients were discharged on the same day with the remaining 2 patients kept overnight due to their procedure being performed late in the day. There were no major adverse events. Minor complications included sore throats lasting less than 48 h [34 of 39 patients (87.1%)] and epigastric pain lasting for a few days [30 of 39 patients (76.9%)]. Interestingly, 3 patients with late dumping syndrome after their original gastric bypass experienced resolution of their postprandial diarrhea. Additionally, at 1 mo follow-up, 8 patients with a history of gastric esophageal reflux noted improvement of their symptoms post-procedure. In terms of weight loss, the average pre-procedure excess body weight was 51.1 kg. Mean weight loss at 2 wk (n = 39) was 3.8 kg (7.4% excess body weight loss, EBWL), at 1 mo (n = 34) was 5.4 kg (10.6% EBWL), at 2 mo (n = 26) was 6.7 kg (13.1% EBWL), at 3 mo (n = 15) was 6.7 kg
(13.1% EBWL), at 6 mo (n = 14) was 8.7 kg (17.0% EBWL) and at 1 year (n = 6) was 10.0 kg (19.5% EBWL).
This trial demonstrates that the StomaphyX™ procedure may offer a safe, effective revisional bariatric procedure; however, long-term randomized prospective studies need to be conducted.
Currently, laparoscopic surgical therapies for morbid obesity are effective in achieving significant weight loss and improving obesity-related co-morbidities over the long-term. However, as is true of all surgical procedures, laparoscopic approaches to weight loss are not without patient restrictions (e.g., multiple co-morbidities, older age, super-obesity, atypical anatomy) and procedural complications[18,19].
Given the persistence of obesity in the United States and limitations of surgical interventions, there is a growing demand for less-invasive approaches. Transoral techniques, as both primary and revisional procedures, are promising in this regard. However, these therapies need to be rigorously tested in a randomized, controlled fashion, to determine their safety and efficacy in both the short- and long-term. In particular, transoral procedures should not only demonstrate equivalent or lower morbidity and mortality rates compared to the current gold-standard therapy (i.e., roux-en-y gastric bypass) but they should also aim to achieve meaningful weight loss and improvement in co-morbid states.
Currently, several aforementioned techniques are being examined in Phase II/III trials (Table 1). If these studies demonstrate that transoral procedures can achieve safe, effective and long-term weight loss, then their applicability could be far reaching. Indeed, the major advantages of transoral techniques include: (1) provision of ambulatory weight loss procedures that may be safer and more cost effective compared with laparoscopic approaches; and (2) circumvention of permanent surgical modification. Therefore, those patients who were precluded for pathological/physiological or financial reasons may be candidates for weight loss procedures. Additionally, transoral techniques may also be used as a bridge for more definitive weight loss procedures. Specifically, using these techniques may provide a way of identifying those patients who are committed to a more definitive surgical intervention. Finally, transoral techniques could provide a safer means of revising bariatric procedures in those individuals that have reached their weight-loss plateau or have started regaining weight.
| Endoluminal vertical gastroplasty | Transoral gastroplasty system | Transoral endoscopic restrictive implant system | Duodenal-jejunal bypass sleeve | Stomaphyx | |
| Phase of development | Phase II | Phase II | Phase I | Phase II/III | Phase III |
| Non-randomized multicenter feasibility studies | Non-randomized multicenter validation trials | Trial still underway-report on first patient | |||
| Patients/trials | n = 82 in 2 trials | n = 32 in 2 trials | n = 20 in 1 trial | n = 37 in 2 trials | n = 39 in 1 trial |
| n = 64[28] | n = 21[31] | n = 12[33] | |||
| n = 18[29] | n = 11[30] | n = 25[34] | |||
| Short term morbidity | Nausea, vomiting, abdominal pain | Nausea, vomiting, epigastric pain, transient dysphagia | Not reported | Nausea, vomiting, abdominal pain | Sore throat, epigastric pain |
| Long term morbidity | Disruption of suture lines (n = 3/64[28]) | Staple line gaps (n = 13/21[31]) | Not reported | Tissue inflammation at site of anchor | None |
| Incomplete distal sleeves (n = 3/21[31]) | |||||
| Mortality | None | None | None | None | None |
| Average length of procedure | 45 min[28] | 160 min[31] | 195 min | 41 min[33] | 35 min |
| 125 min[29] | 84 min[30] | 39 min[34] | |||
| Tested in randomized trials | No | No | No | Yes[34] | Yes |
| %EWL | Not reported | ||||
| 1 mo | 21.1[28] | 16.2[31]; 19.2[30] | 16.0[33]; 15.0[34] | 10.6 | |
| 3 mo | 39.6[28] | 22.6[31]; 33.7[30] | 21.0 | 24.0[33]; 21.0[34] | 13.1 |
| 6 mo | Not reported | 24.4[31]; 46.0[30] | 26.0 | Not reported | 17.0 |
| 12 mo | 58.1 (n = 59/64)[28] | Not reported | Not reported | Not reported | 19.5 |
Transoral techniques are rapidly becoming a reality in the armamentarium of weight loss procedures and it is our responsibility to ensure that these techniques are safe and effective in the long-term.
Peer reviewers: Adam Donald Farmer, Dr., Wingate Institute of Neurogastroenterology, 26 Ashfield Street, London E1 2AJ, United Kingdom; Perminder Phull, MD, FRCP, FRCPE, Gastrointestinal and Liver Service, Room 2.58, Ashgrove House, Aberdeen Royal Infirmary, Foresterhill, Aberdeen AB25 2ZN, United Kingdom
S- Editor Zhang SJ L- Editor Roemmele A E- Editor Zheng XM
| 1. | World Health Organization. Fact sheet: obesity and overweight. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/print.html. |
| 2. | Ogden CL. Disparities in obesity prevalence in the United States: black women at risk. Am J Clin Nutr. 2009;89:1001-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6297] [Cited by in RCA: 5884] [Article Influence: 309.7] [Reference Citation Analysis (0)] |
| 4. | Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity among adults in the United States--no statistically significant chance since 2003-2004. NCHS Data Brief. 2007;1-8. [PubMed] |
| 5. | Bessesen DH. Update on obesity. J Clin Endocrinol Metab. 2008;93:2027-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | World Health Organization. World Health Report 2002. Available from: http://www.iotf.org. |
| 7. | Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 2879] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 8. | Overweight, obesity, and health risk. National Task Force on the Prevention and Treatment of Obesity. Arch Intern Med. 2000;160:898-904. [PubMed] |
| 9. | Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1546] [Cited by in RCA: 1428] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 10. | Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763-778. [PubMed] |
| 11. | Serdula MK, Mokdad AH, Williamson DF, Galuska DA, Mendlein JM, Heath GW. Prevalence of attempting weight loss and strategies for controlling weight. JAMA. 1999;282:1353-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 315] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | North American Association for the Study of Obesity. The Practical Guide: Identification, evaluation, and treatment of overweight and obesity in adults. Bethesda, Md: National Institutes of Health 2000; NIH publication 00-4084 Available from: http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. |
| 13. | NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956-961. [PubMed] |
| 14. | Gastrointestinal surgery for severe obesity. Proceedings of a National Institutes of Health Consensus Development Conference. March 25-27, 1991, Bethesda, MD. Am J Clin Nutr. 1992;55:487S-619S. [PubMed] |
| 15. | Parker M, Loewen M, Sullivan T, Yatco E, Cerabona T, Savino JA, Kaul A. Predictors of outcome after obesity surgery in New York state from 1991 to 2003. Surg Endosc. 2007;21:1482-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5073] [Cited by in RCA: 4703] [Article Influence: 224.0] [Reference Citation Analysis (1)] |
| 17. | Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery. 2007;142:621-32; discussion 632-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 445] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 18. | Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, Nguyen NT, Li Z, Mojica WA, Hilton L. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547-559. [PubMed] |
| 19. | Allen JW. Laparoscopic gastric band complications. Med Clin North Am. 2007;91:485-97, xii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev. 2009;CD003641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 21. | Hazey JW, Dunkin BJ, Melvin WS. Changing attitudes toward endolumenal therapy. Surg Endosc. 2007;21:445-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Mathus-Vliegen EM, Tytgat GN. Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. Gastrointest Endosc. 2005;61:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Genco A, Bruni T, Doldi SB, Forestieri P, Marino M, Busetto L, Giardiello C, Angrisani L, Pecchioli L, Stornelli P. BioEnterics Intragastric Balloon: The Italian Experience with 2,515 Patients. Obes Surg. 2005;15:1161-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Genco A, Cipriano M, Bacci V, Cuzzolaro M, Materia A, Raparelli L, Docimo C, Lorenzo M, Basso N. BioEnterics Intragastric Balloon (BIB): a short-term, double-blind, randomised, controlled, crossover study on weight reduction in morbidly obese patients. Int J Obes (Lond). 2006;30:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Schwartz MP, Wellink H, Gooszen HG, Conchillo JM, Samsom M, Smout AJ. Endoscopic gastroplication for the treatment of gastro-oesophageal reflux disease: a randomised, sham-controlled trial. Gut. 2007;56:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Mahmood Z, McMahon BP, Arfin Q, Byrne PJ, Reynolds JV, Murphy EM, Weir DG. Endocinch therapy for gastro-oesophageal reflux disease: a one year prospective follow up. Gut. 2003;52:34-39. [PubMed] |
| 27. | Rothstein RI, Filipi CJ. Endoscopic suturing for gastroesophageal reflux disease: clinical outcome with the Bard EndoCinch. Gastrointest Endosc Clin N Am. 2003;13:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Fogel R, De Fogel J, Bonilla Y, De La Fuente R. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc. 2008;68:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Brethauer SA, Chand B, Schauer PR, Thompson CC. Transoral gastric volume reduction for weight management: technique and feasibility in 18 patients. Surg Obes Relat Dis. 2010;6:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Devière J, Ojeda Valdes G, Cuevas Herrera L, Closset J, Le Moine O, Eisendrath P, Moreno C, Dugardeyn S, Barea M, de la Torre R. Safety, feasibility and weight loss after transoral gastroplasty: First human multicenter study. Surg Endosc. 2008;22:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Moreno C, Closset J, Dugardeyn S, Baréa M, Mehdi A, Collignon L, Zalcman M, Baurain M, Le Moine O, Devière J. Transoral gastroplasty is safe, feasible, and induces significant weight loss in morbidly obese patients: results of the second human pilot study. Endoscopy. 2008;40:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Biertho L, Hould FS, Lebel S, Biron S. Transoral endoscopic restrictive implant system: a new endoscopic technique for the treatment of obesity. Surg Obes Relat Dis. 2010;6:203-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Rodriguez-Grunert L, Galvao Neto MP, Alamo M, Ramos AC, Baez PB, Tarnoff M. First human experience with endoscopically delivered and retrieved duodenal-jejunal bypass sleeve. Surg Obes Relat Dis. 2008;4:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Tarnoff M, Rodriguez L, Escalona A, Ramos A, Neto M, Alamo M, Reyes E, Pimentel F, Ibanez L. Open label, prospective, randomized controlled trial of an endoscopic duodenal-jejunal bypass sleeve versus low calorie diet for pre-operative weight loss in bariatric surgery. Surg Endosc. 2009;23:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Yale CE. Gastric surgery for morbid obesity. Complications and long-term weight control. Arch Surg. 1989;124:941-946. [PubMed] |
| 36. | Sugerman HJ, Kellum JM, Engle KM, Wolfe L, Starkey JV, Birkenhauer R, Fletcher P, Sawyer MJ. Gastric bypass for treating severe obesity. Am J Clin Nutr. 1992;55:560S-566S. [PubMed] |
| 37. | Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 499] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 38. | Martin MJ, Mullenix PS, Steele SR, See CS, Cuadrado DG, Carter PL. A case-match analysis of failed prior bariatric procedures converted to resectional gastric bypass. Am J Surg. 2004;187:666-70; discussion 670-1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Mikami D, Needleman B, Narula V, Durant J, Melvin WS. Natural orifice surgery: initial US experience utilizing the StomaphyX device to reduce gastric pouches after Roux-en-Y gastric bypass. Surg Endosc. 2010;24:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |