Published online May 8, 2017. doi: 10.4254/wjh.v9.i13.635
Peer-review started: January 23, 2017
First decision: February 17, 2017
Revised: March 14, 2017
Accepted: April 6, 2017
Article in press: April 10, 2017
Published online: May 8, 2017
To evaluate the significance of resection margin width in the management of hepatocholangiocarcinoma (HCC-CC).
Data of consecutive patients who underwent hepatectomy for hepatic malignancies in the period from 1995 to 2014 were reviewed. Patients with pathologically confirmed HCC-CC were included for analysis. Demographic, biochemical, operative and pathological data were analyzed against survival outcomes.
Forty-two patients were included for analysis. The median age was 53.5 years. There were 29 males. Hepatitis B virus was identified in 73.8% of the patients. Most patients had preserved liver function. The median preoperative indocyanine green retention rate at 15 min was 10.2%. The median tumor size was 6.5 cm. Major hepatectomy was required in over 70% of the patients. Hepaticojejunostomy was performed in 6 patients. No hospital death occurred. The median hospital stay was 13 d. The median follow-up period was 32 mo. The 5-year disease-free survival and overall survival were 23.6% and 35.4% respectively. Multifocality was the only independent factor associated with disease-free survival [P < 0.001, odds ratio 4, 95% confidence interval (CI): 1.9-8.0]. In patients with multifocal tumor (n = 20), resection margin of ≥ 1 cm was associated with improved 1-year disease-free survival (40% vs 0%; log-rank, P = 0.012).
HCC-CC is a rare disease with poor prognosis. Resection margin of 1 cm or above was associated with improved survival outcome in patients with multifocal HCC-CC.
Core tip: A retrospective review of all patients who had undergone curative resection for hepatocholangiocarcinoma in the last 20 years was performed in a university center. The 5-year disease-free and overall survival were 23.6% and 35.4% respectively. Various patient and disease factors were investigated with respect to their effect to disease free and overall survival using cox regression analysis. Multifocality was the only independent factor associated with disease-free survival (P < 0.001). In a subgroup of patient (n = 20) who had multifocal tumor, resection margin of ≥ 1 cm was associated with improved 1-year disease-free survival (40% vs 0%, P = 0.012).
- Citation: Ma KW, Chok KSH. Importance of surgical margin in the outcomes of hepatocholangiocarcinoma. World J Hepatol 2017; 9(13): 635-641
- URL: https://www.wjgnet.com/1948-5182/full/v9/i13/635.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i13.635
Hepatocholangiocarcinoma (HCC-CC) is a rare disease entity contributing to 1%-3% of primary hepatic malignancies[1-4]. Histologically, tumor cells of hepatocyte and bile ductal epithelial origins are identified in HCC-CC[5]. While “pseudoglandular” structures can as well be observed in other hepatocellular carcinoma (HCC) variants[6], genuine HCC-CC should demonstrate true glandular structures with mucin production[7]. Since the first description of HCC-CC in 1949 by Allen and Lisa[8], 3 subtypes of the disease were established: Type 1, double separate tumors - HCC and intrahepatic cholangiocarcinoma (ICC) - in the same liver; type 2, the presence of HCC and ICC in a continuum; type 3, intermingling of HCC and ICC cells[8]. In 1985, Goodman et al[9] revised the classification with new descriptions of 3 types of HCC-CC: The collision type, the transitional type, and fibrolamellar HCC with mucin-producing pseudoglands. Later, the World Health Organization redefined HCC-CC as a distinct tumor with intimate and unequivocal fusion of HCC and ICC cells[10]. The disease’s clinical outcomes and prognostic factors have barely been studied. The median survival after HCC-CC resection varied from study to study, from 12 to 48 mo[11-15]. This disparity may be partially explained by the heterogeneity in diagnostic criteria for HCC-CC in the studies. The inclusion of HCC variants (which do not contain genuine ICC components) and the collision type of HCC-CC (which is no longer regarded as HCC-CC according to the World Health Organization) probably led to data contamination and resulted in difference in prognosis[16].
The width of resection margin had been shown to affect the oncological outcomes of hepatectomy for HCC[17-19] and ICC[20,21]. In a prospective randomized trial involving 169 patients by Shi et al[19], patients who were randomized to the narrow margin group (1 cm) had significantly inferior 5-year overall survival when compared with patients who had HCC resection with wide margin (2 cm) (49.1% vs 74.9%). For the role of resection margin in ICC, Farges et al[21] demonstrated a significant correlation between resection margin and median survival in a subgroup of node-negative patients (≤ 1 mm: 15 mo, 2-4 mm: 36 mo, 5-9 mm: 57 mo, ≥ 10 mm: 64 mo; P < 0.001). In a recent article by our center, patients with early ICC were shown to benefit from resection margin of over 1 cm[20]. Nonetheless, the role of resection margin in management of HCC-CC remains to be defined. This retrospective study aimed to elucidate the clinical features of HCC-CC and the impact of resection margin width on patient survival.
Data of consecutive patients who underwent hepatectomy for hepatic malignancies in the period from 1995 to 2014 were reviewed. Patients included for analysis were those who: (1) had pathologically confirmed HCC-CC; (2) were not younger than 18 years; and (3) did not receive re-resection for recurrent HCC-CC. Diagnosis of HCC-CC was made by a combination of histological and immunohistochemical staining[22,23], supplemented by electron microscopy examination when necessary[11]. Demographic, biochemical, operative and pathological data were analyzed against survival outcomes. Categorical parameters were analyzed with Pearson’s χ2 test and continuous data were analyzed with the Mann-Whitney U test. Univariate analysis with bivariate correlation and multivariate analysis with the Cox regression model were performed. In this study, survival outcomes of HCC-CC were compared with the HCC and ICC patients of the same period. The Kaplan-Meier method was used for survival analysis and the log-rank test was used for survival comparison. P-values of ≤ 0.05 were considered statistically significant. The computer software Statistical Product and Service Solutions for Windows (SPSS, Chicago, Illinois, United States) was used for statistical analyses.
Before hepatectomy, a basic biochemistry test was performed to assess complete blood picture, clotting profile, and liver and renal functions. Levels of tumor markers such as alpha-fetoprotein, carcinoembryonic antigen and cancer antigen 19-9 were recorded. Major hepatectomy was defined as resection of more than 3 Couinaud segments. Indocyanine green retention rate at 15 min after injection (ICG-R15) was used to evaluate the sufficiency of liver function for hepatectomy. For major hepatectomy, ICG-R15 of ≤ 18% was required. For minor hepatectomy, ICG-R15 of ≤ 22% was required. Patients having planned major hepatectomy were required to undergo computed tomographic volumetric study. The minimum ratio of future liver remnant to standard liver volume was 25% for non-cirrhotic livers[24,25]. Our technique of liver resection has been described elsewhere[24]. For follow-up, patients were seen at our out-patient clinic every 3 mo in the first 2 years and every 6 mo afterwards. Tumor markers were checked in every visit. Computed tomographic scan was performed 1-3 mo after discharge and then every 6 mo. Adjuvant therapy was not a routine and was offered at the discretion of the surgeon. Recurrence was defined as the presence of radiological or histological evidence of intrahepatic or extrahepatic HCC-CC.
From 1995 to 2014, 1696 patients underwent hepatectomy for primary liver malignancy. Among them, 50 adult patients had pathologically confirmed HCC-CC (3%). Eight of these 50 patients were excluded because of re-resection. As a result, 42 patients were included for analysis. Their demographic characteristics and baseline biochemistry are shown in Table 1.
| No. of patients = 42 | |
| Male:female | 29:13 |
| Age (yr) | 52.5 (26-72) |
| Hepatitis B virus carrier | 31 (73.8%) |
| Hepatitis C virus carrier | 0 |
| Hemoglobin (g/dL) | 13.4 (8.6-16.7) |
| White cell count (10 × 6/L) | 5.8 (3.5-10.1) |
| Platelet count (10 × 9/L) | 185 (89-499) |
| Creatinine (mmol/L) | 84 (61-131) |
| Total bilirubin (mmol/L) | 10 (2-61) |
| Albumin (g/L) | 40 (29-49) |
| Aspartate transaminase (umol/L) | 44 (14-270) |
| Alkaline phosphatase (umol/L) | 92 (26-516) |
| Prothrombin time (s) | 13.5 (10.9-13.5) |
| Alpha-fetoprotein (u/L) | 75.5 (2-219020) |
| Carcinoembryonic antigen (u/L) | 2.3 (0.4-5.9) |
Most of the patients required major hepatectomy, and right hepatectomy was the most commonly performed procedure. Hepaticojejunostomy was performed in 6 patients (Table 2). The median operation time was 414 min (range, 177-1149 min) and the median blood loss volume was 800 mL (range, 5-2400 mL). There was no hospital death. The median length of hospital stay was 13 d (range, 3-50 d). Three patients developed postoperative complications of Clavien-Dindo grade 3a or above (grade 3a in 1 patient and grade 4 in 2 patients).
| No. of patients (%) | |
| Right/extended right hepatectomy | 17 (40.5) |
| Left/extended left hepatectomy | 5 (11.9) |
| Right trisectionectomy | 6 (14.3) |
| Left trisectionectomy | 1 (2.4) |
| Central bisectionectomy | 2 (4.8) |
| Left lateral sectionectomy | 3 (7.1) |
| Other minor hepatectomy | 8 (19.0) |
Histological examination was performed for all patients. The median tumor size was 6.5 cm (range, 2-23 cm). Twenty patients (47.6%) had multiple (more than 1) tumor nodules. Moderate tumor differentiation (new Edmondson grading) was found in 40% of the patients and 33.3% of the patients had poor tumor differentiation. R0 resection was achieved in 90% of the patients. The median resection margin width was 1 cm (range, 0-6 cm).
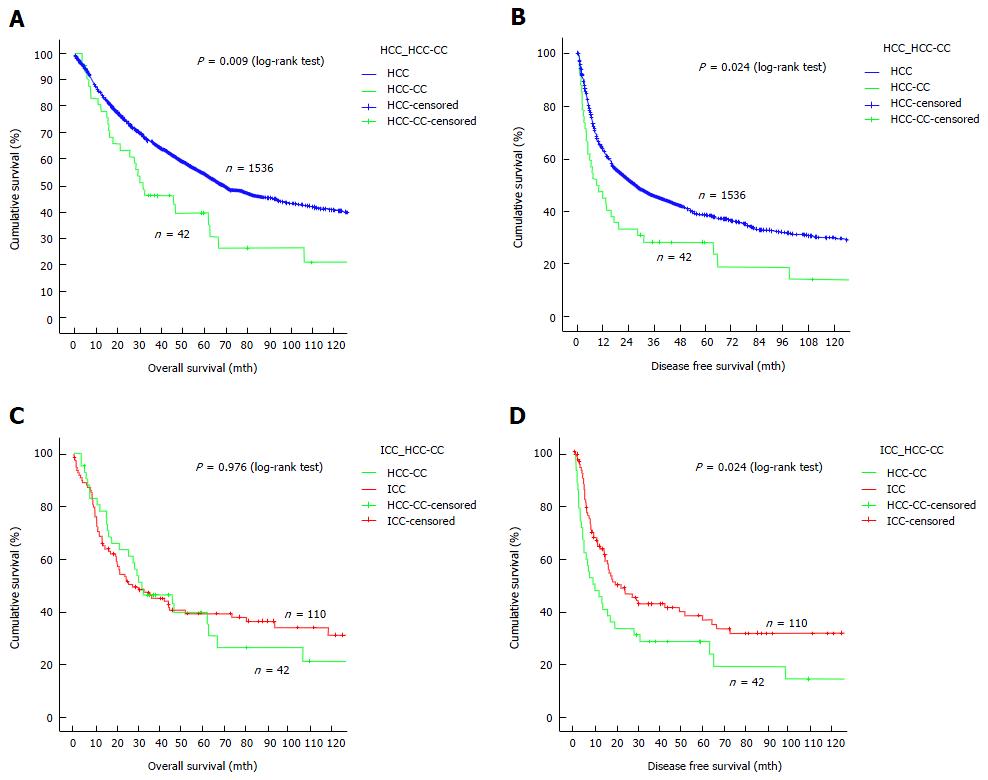
The median follow-up period was 110 mo. Adjuvant treatment was given to 13 patients in the form of transarterial chemo- or radio-embolization, systemic chemotherapy, external radiotherapy, molecular targeted therapy, or a combination of any of these. When it comes to survival outcomes, HCC-CC patients compared unfavorably with HCC patients. The median overall survival was 32 mo in HCC-CC patients and 70 mo in HCC patients (Figure 1A), and the median disease-free survival was 9 mo in the former and 28 mo in the latter (Figure 1B). On the other hand, HCC-CC patients and ICC patients had comparable overall survival (a median of 27 mo in ICC patients) (Figure 1C) while the latter had better disease-free survival (median, 20 mo) (Figure 1D). Recurrence developed in 33 HCC-CC patients (78.6%) (14 had intrahepatic recurrence, 3 had extrahepatic recurrence, and 16 had both).
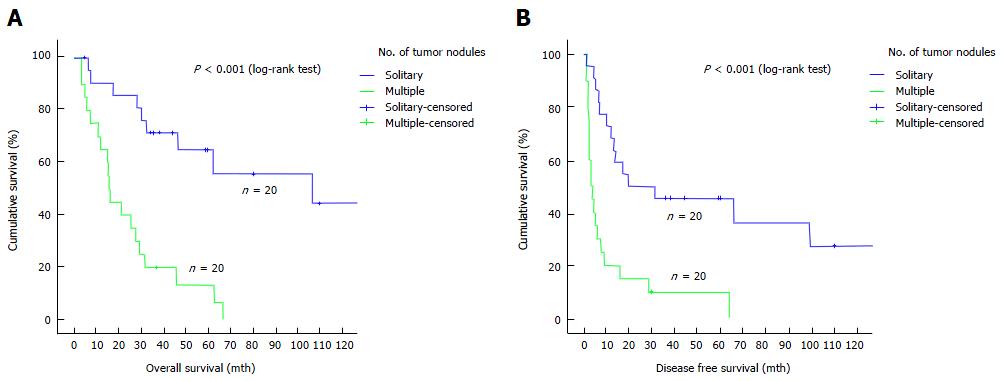
In Cox regression analysis, tumor multiplicity was the only independent factor associated with overall survival [P < 0.001, odds ratio (OR) 5.26, 95%CI: 2.254-12.290] and disease-free survival (P = 0.001, OR 4.00, 95%CI: 1.897-8.434) (Table 3). Patients with solitary tumor nodule had a median overall survival of 106 mo whereas those with multiple tumor nodules had a median overall survival of 16 mo (P < 0.001) (Figure 2A). The median disease-free survival was 19.2 mo in patients with solitary tumor nodule and 3.1 mo in patients with multiple tumor nodules (P < 0.001) (Figure 2B).
| Factor | Overall survival (P-value) | Disease-free survival (P-value) | ||
| Univariate | Multivariate | Univariate | Multivariate | |
| Age | 0.269 | NS | 0.501 | NS |
| Sex | 0.513 | NS | 0.868 | NS |
| HBV status | 0.507 | NS | 0.441 | NS |
| Platelet count | 0.389 | NS | 0.331 | NS |
| Total bilirubin | 0.471 | NS | 0.176 | NS |
| Albumin | 0.811 | NS | 0.663 | NS |
| ICG-R15 | 0.955 | NS | 0.749 | NS |
| AFP | 0.937 | NS | 0.308 | NS |
| CEA | 0.832 | NS | 0.716 | NS |
| Operation time | 0.239 | NS | 0.682 | NS |
| Blood loss | 0.138 | NS | 0.037 | NS |
| Resection extent1 | 0.152 | NS | 0.108 | NS |
| Tumor size | 0.845 | NS | 0.975 | NS |
| Multifocality | < 0.0001 | < 0.001 | < 0.0001 | 0.001 |
| Margin width | 0.523 | NS | 0.9 | NS |
| Wide margin (≥ 1 cm) | 0.491 | NS | 0.096 | NS |
| Microvascular invasion | 0.373 | NS | 0.170 | NS |
| Nodal metastasis | 0.314 | NS | 0.229 | NS |
| Adjuvant treatment | 0.162 | NS | 0.052 | NS |
Further analyses of the subgroup of patients (n = 20) who had multiple tumor nodules were performed. In univariate analysis, disease-free survival had an association with preoperative albumin level (P = 0.022) and resection margin width (P = 0.013). Multivariate analysis showed that resection margin width was the only independent factor affecting disease-free survival. A clear resection margin of ≥ 1 cm could improve 1-year disease-free survival from 0% to 40% (P = 0.012) (Figure 3).
This retrospective study has further illustrated that HCC-CC is a rare and sinister primary hepatic malignancy. The reported incidences of HCC-CC vary greatly. This is probably due to the difference in the pathological definition of the disease. HCC-CC shares the clinicopathological features of HCC and ICC. Male predominance, the existence of background cirrhosis and elevation of alpha-fetoprotein level are hallmarks of HCC. These features are also often seen in HCC-CC. Tumor hypovascularity, involvement of regional lymphadenopathy and poor survival outcomes are common in HCC-CC as well as ICC. This study found that HCC-CC patients had significantly worse overall survival and disease-free survival when compared with HCC patients, which concurs with other reports[26-29]. When compared with ICC patients, HCC-CC patients had inferior disease-free survival but were comparable in overall survival. This explains why HCC-CC should be included in the section of carcinoma of the intrahepatic duct in the 7th edition of the AJCC cancer staging manual[30]. The worse survival outcomes were attributable to its propensity for vascular invasion and lymph node metastasis[1,9,31].
Despite the availability of the various classification systems for HCC-CC[8,9,32], its prognosis remains difficult. Chantajitr et al[33] reported that a cancer antigen 19-9 level of ≥ 80 u/mL and the presence of intrahepatic ductal dilatation were independent factors for poor survival. Other studies found that lymphovascular permeation, large tumor size and the presence of tumor satellites were poor prognostic factors[4,34-37]. In the current study, tumor multiplicity was the only independent factor associated with inferior disease-free survival and overall survival. This echoes the emphasis on the significance of tumor multiplicity in the staging of ICC in the 7th edition of the AJCC Staging[30]. The role of adjuvant therapy in HCC-CC management is still unclear. One fourth of the patients in the current study received some form of adjuvant treatment (transarterial chemoembolization, radiotherapy, systemic therapy, etc.) at the discretion of the surgeon. Standardization of adjuvant treatment protocol is necessary before the role of adjuvant therapy can be established.
The current study could not demonstrate any benefit of R0 resection for patients with resectable HCC-CC, probably because of the small number of patients with R1 or R2 resection. Since HCC-CC is intrinsically associated with poorer prognostic outcomes when compared with HCC and ICC, small survival advantage conferred by wide resection margin (1 cm or above) could only be shown with a larger study population. However, this survival benefit was demonstrated in the subgroup of patients who had multifocal disease (40% vs 0% disease-free survival at 1 year). Since HCC-CC inherits the tumor biology of HCC and ICC, it has the ability of portal vein invasion and lymphovascular permeation. We therefore postulate that wide resection or even routine anatomical resection would eliminate residual satellite tumor cells or microtumor residing in the same vasculobiliary territory, thereby improving disease-free survival. The retrospective nature of the current study has posed a couple of limitations. Firstly, missing data on carbohydrate antigen 19-9 made adequate analysis of its influence on survival outcomes impossible. In most of the cases, HCC-CC was diagnosed as HCC and routine blood check for carbohydrate antigen 19-9 was clinically irrelevant. Secondly, the small cohort size predisposed the study to type-II error; some potentially significant factors related to survival outcomes might not be identified by the analysis. However, the study period spanned two decades (1995-2014), which is relatively long. Furthermore, survival comparison between the study cohort and two much larger groups of patients (1536 HCC patients and 110 ICC patients) was performed, which should provide important data reference for future research.
HCC-CC is a rare and sinister primary hepatic malignancy. Patients with solitary tumor had better survival. A resection margin of at least 1 cm improved the disease-free survival of patients with multiple tumor nodules.
Hepatocholangiocarcinoma (HCC-CC) is an uncommon primary hepatic malignancy, contributing to about 1%-3% of all primary liver cancers. Its prognosis is worse than hepatocellular carcinoma (HCC) and similar to that of the intrahepatic cholangiocarcinoma. While resection margin was found to be an important factor associated with long-term oncological outcomes, its role in the management of this rare entity has not been reported.
The role of resection margin has been extensively investigated in many cancers, such as oesophageal and colorectal cancers. In HCC and intrahepatic cholangiocarcinoma, wide resection margin was shown to be an independent factor leading to improved survival outcomes. In the context of HCC-CC, previous reports focused mainly on the epidemiology, diagnosis and disease nature, yet, the role of resection margin remained an unexplored area of the disease.
The rarity of the disease has always been a hurdle for statistical analysis. With the use of a well-maintained patient database in a university surgical center, a HCC-CC population of relatively large sample size were retrieved for analysis.
The results of this study showed that HCC-CC is associated with significantly worse overall survival when compared to HCC (9 mo vs 28 mo). Multifocality was found to be the only independent factor associated with inferior disease free survival. Early and regular postoperative surveillance should be offered to this group of patients for early detection of recurrence. In patients with multifocal HCC-CC, attempt should be made to achieve a clear resection margin of 1cm so as to improve the recurrence free survival.
HCC-CC is a rare disease condition and histologically, the features of HCC and cholangiocarcinoma should both be demonstrated in the same tumour mass according to World Health Organization criteria.
This article is important for clinical management of HCC-CC, with well-designed analysis and trustable conclusions.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chuang WL, Wang H, Xiao J S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277-287. [PubMed] [Cited in This Article: ] |
| 2. | Aoki K, Takayasu K, Kawano T, Muramatsu Y, Moriyama N, Wakao F, Yamamoto J, Shimada K, Takayama T, Kosuge T. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features and computed tomographic findings. Hepatology. 1993;18:1090-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 77] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Wachtel MS, Zhang Y, Xu T, Chiriva-Internati M, Frezza EE. Combined hepatocellular cholangiocarcinomas; analysis of a large database. Clin Med Pathol. 2008;1:43-47. [PubMed] [Cited in This Article: ] |
| 4. | Tang D, Nagano H, Nakamura M, Wada H, Marubashi S, Miyamoto A, Takeda Y, Umeshita K, Dono K, Monden M. Clinical and pathological features of Allen’s type C classification of resected combined hepatocellular and cholangiocarcinoma: a comparative study with hepatocellular carcinoma and cholangiocellular carcinoma. J Gastrointest Surg. 2006;10:987-998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Molmenti EP, Marsh JW, Dvorchik I, Oliver JH, Madariaga J, Iwatsuki S. Hepatobiliary malignancies. Primary hepatic malignant neoplasms. Surg Clin North Am. 1999;79:43-57, viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Passmore R. LIVER: STRUCTURE AND FUNCTION. By H. Popper and F. Schaffner. New York, Toronto and London: McGraw-Hill Book Company Inc. 1957. Pp. xv 777. Q J Exp Physiol Cogn Med Sci. 1957;42:418-419. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 7. | Anthony PP. Primary carcinoma of the liver: a study of 282 cases in Ugandan Africans. J Pathol. 1973;110:37-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 159] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647-655. [PubMed] [Cited in This Article: ] |
| 9. | Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55:124-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 10. | Ishak K, Anthony P, Sobin L. Histological typing of tumours of the liver. WHO International Histological Classification of Tumours. World Health Orgnization 2nd edn. Springer, Berlin Heidelberg NewYork. 1994;17-19. [DOI] [Cited in This Article: ] |
| 11. | Ng IO, Shek TW, Nicholls J, Ma LT. Combined hepatocellular-cholangiocarcinoma: a clinicopathological study. J Gastroenterol Hepatol. 1998;13:34-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH, Klimstra D. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040-2046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 244] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Nakamura S, Suzuki S, Sakaguchi T, Serizawa A, Konno H, Baba S, Baba S, Muro H. Surgical treatment of patients with mixed hepatocellular carcinoma and cholangiocarcinoma. Cancer. 1996;78:1671-1676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 14. | Liu CL, Fan ST, Lo CM, Ng IO, Lam CM, Poon RT, Wong J. Hepatic resection for combined hepatocellular and cholangiocarcinoma. Arch Surg. 2003;138:86-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Chok KS, Ng KK, Cheung TT, Yuen WK, Poon RT, Lo CM, Fan ST. An update on long-term outcome of curative hepatic resection for hepatocholangiocarcinoma. World J Surg. 2009;33:1916-1921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Yeh MM. Pathology of combined hepatocellular-cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25:1485-1492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Eguchi S, Kanematsu T, Arii S, Okazaki M, Okita K, Omata M, Ikai I, Kudo M, Kojiro M, Makuuchi M. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008;143:469-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 298] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 18. | European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4059] [Cited by in F6Publishing: 4345] [Article Influence: 362.1] [Reference Citation Analysis (2)] |
| 19. | Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, Lau WY, Li JQ. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245:36-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 374] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 20. | Ma KW, Cheung TT, She WH, Chok KS, Chan AC, Ng IO, Chan SC, Lo CM. The effect of wide resection margin in patients with intrahepatic cholangiocarcinoma: A single-center experience. Medicine (Baltimore). 2016;95:e4133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Farges O, Fuks D, Boleslawski E, Le Treut YP, Castaing D, Laurent A, Ducerf C, Rivoire M, Bachellier P, Chiche L. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254:824-829; discussion 830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 22. | Hurlimann J, Gardiol D. Immunohistochemistry in the differential diagnosis of liver carcinomas. Am J Surg Pathol. 1991;15:280-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 107] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Ferrandez-Izquierdo A, Llombart-Bosch A. Immunohistochemical characterization of 130 cases of primary hepatic carcinomas. Pathol Res Pract. 1987;182:783-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 542] [Cited by in F6Publishing: 552] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 25. | Chan SC, Liu CL, Lo CM, Lam BK, Lee EW, Wong Y, Fan ST. Estimating liver weight of adults by body weight and gender. World J Gastroenterol. 2006;12:2217-2222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 55] [Cited by in F6Publishing: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Wang AQ, Zheng YC, Du J, Zhu CP, Huang HC, Wang SS, Wu LC, Wan XS, Zhang HH, Miao RY. Combined hepatocellular cholangiocarcinoma: Controversies to be addressed. World J Gastroenterol. 2016;22:4459-4465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 37] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Yoon YI, Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, Song GW, Jung DH, Lee JW. Postresection Outcomes of Combined Hepatocellular Carcinoma-Cholangiocarcinoma, Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Gastrointest Surg. 2016;20:411-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Lee JH, Chung GE, Yu SJ, Hwang SY, Kim JS, Kim HY, Yoon JH, Lee HS, Yi NJ, Suh KS. Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol. 2011;45:69-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Lee WS, Lee KW, Heo JS, Kim SJ, Choi SH, Kim YI, Joh JW. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today. 2006;36:892-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5537] [Cited by in F6Publishing: 6112] [Article Influence: 436.6] [Reference Citation Analysis (0)] |
| 31. | Uenishi T, Hirohashi K, Shuto T, Yamamoto T, Kubo S, Tanaka H, Ikebe T, Kinoshita H. Surgery for mixed hepatocellular and cholangiocellular carcinoma. Hepatogastroenterology. 2000;47:832-834. [PubMed] [Cited in This Article: ] |
| 32. | The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg. 1989;19:98-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 253] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Chantajitr S, Wilasrusmee C, Lertsitichai P, Phromsopha N. Combined hepatocellular and cholangiocarcinoma: clinical features and prognostic study in a Thai population. J Hepatobiliary Pancreat Surg. 2006;13:537-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, Rhee JC, Cho JW, Park CK, Kim HJ. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | Yano Y, Yamamoto J, Kosuge T, Sakamoto Y, Yamasaki S, Shimada K, Ojima H, Sakamoto M, Takayama T, Makuuchi M. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol. 2003;33:283-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Sanada Y, Shiozaki S, Aoki H, Takakura N, Yoshida K, Yamaguchi Y. A clinical study of 11 cases of combined hepatocellular-cholangiocarcinoma Assessment of enhancement patterns on dynamics computed tomography before resection. Hepatol Res. 2005;32:185-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Shin CI, Lee JM, Kim SH, Choi JY, Lee JY, Han JK, Jo SY, Choi BI. Recurrence patterns of combined hepatocellular-cholangiocarcinoma on enhanced computed tomography. J Comput Assist Tomogr. 2007;31:109-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |