Published online Apr 28, 2016. doi: 10.4254/wjh.v8.i12.566
Peer-review started: January 14, 2016
First decision: February 2, 2016
Revised: February 28, 2016
Accepted: April 7, 2016
Article in press: April 11, 2016
Published online: April 28, 2016
AIM: To determine the predictors of 50 d in-hospital mortality in decompensated cirrhosis patients with spontaneous bacterial peritonitis (SBP).
METHODS: Two hundred and eighteen patients admitted to an intensive care unit in a tertiary care hospital between June 2013 and June 2014 with the diagnosis of SBP (during hospitalization) and cirrhosis were retrospectively analysed. SBP was diagnosed by abdominal paracentesis in the presence of polymorphonuclear cell count ≥ 250 cells/mm3 in the peritoneal fluid. Student’s t test, multivariate logistic regression, cox proportional hazard ratio (HR), receiver operating characteristics (ROC) curves and Kaplan-Meier survival analysis were utilized for statistical analysis. Predictive abilities of several variables identified by multivariate analysis were compared using the area under ROC curve. P < 0.05 were considered statistical significant.
RESULTS: The 50 d in-hospital mortality rate attributable to SBP is 43.11% (n = 94). Median survival duration for those who died was 9 d. In univariate analysis acute kidney injury (AKI), hepatic encephalopathy, septic shock, serum bilirubin, international normalized ratio, aspartate transaminase, and model for end-stage liver disease - sodium (MELD-Na) were significantly associated with in - hospital mortality in patients with SBP (P≤ 0.001). Multivariate cox proportional regression analysis showed AKI (HR = 2.16, 95%CI: 1.36-3.42, P = 0.001) septic shock (HR = 1.73, 95%CI: 1.05-2.83, P = 0.029) MELD-Na (HR = 1.06, 95%CI: 1.02-1.09, P≤ 0.001) was significantly associated with 50 d in-hospital mortality. The prognostic accuracy for AKI, MELD-Na and septic shock was 77%, 74% and 71% respectively associated with 50 d in-hospital mortality in SBP patients.
CONCLUSION: AKI, MELD-Na and septic shock were predictors of 50 d in-hospital mortality in decompensated cirrhosis patients with SBP.
Core tip: Spontaneous bacterial peritonitis (SBP) is associated with poor prognosis especially with in-hospital patients. The mortality rate ranges from 20%-40%. The model for end-stage liver disease (MELD) has been suggested as a predictor of the in-hospital mortality in patients with SBP. However, the role of other predictors has not been established. The goal of this study is to identify other prognostic factors for mortality in decompensated cirrhotic patients with SBP and to evaluate the predictive power of acute kidney injury, MELD-sodium and septic shock.
- Citation: Bal CK, Daman R, Bhatia V. Predictors of fifty days in-hospital mortality in decompensated cirrhosis patients with spontaneous bacterial peritonitis. World J Hepatol 2016; 8(12): 566-572
- URL: https://www.wjgnet.com/1948-5182/full/v8/i12/566.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i12.566
Spontaneous bacterial peritonitis (SBP) is defined as acute infection of ascitic fluid without any identifiable surgically treatable intra-abdominal source[1]. SBP is a major complication of decompensated cirrhosis with ascites[2]. The SBP is diagnosed by abdominal paracentesis with an elevated ascitic fluid neutrophil count (≥ 250 cells/mm3) and/or a positive ascitic fluid culture. In up to 60% cases, gram-negative bacteria (Escherichia coli or Klebsiella pneumonia) are most prevalent organism involved[3]. In about 25% of the cases, gram-positive bacteria (mainly Streptococcus species and Enterococci) are involved[3]. The prevalence of SBP is up to 30% in hospitalized cirrhotic patients with ascites[4]. Despite intensive management, the in-hospital mortality remains between 20%-40%[5]. Model for end-stage liver disease (MELD) scores have been investigated with their predictive accuracy; however it is vulnerable to variations in laboratory measurements, making their utilization in prediction of SBP related in-hospital mortality affected[6,7]. In addition, decompensated cirrhotics with major complication like SBP may have low MELD scores with high mortality[7]. Acute kidney injury (AKI) is common in patients with decompensated cirrhosis with ascites. AKI in cirrhosis was diagnosed by AKI network (AKIN) based on serum creatinine/urine output. AKI can be used to predict mortality in decompensated cirrhotic with ascites[8]. SBP-associated septic shock carries significant mortality in cirrhosis[9]. Thus this study aimed to have a comprehensive approach to determine possible prognostic factors predicting SBP related in-hospital mortality, compare the predictive power of AKI, MELD-sodium (MELD-Na), and septic shock and to identify the best cut-off point of MELD-Na scores to predict 50 d in-hospital mortality.
Medical records of 218 adult patients admitted to hepatology intensive care unit (ICU) of the Institute of Liver and Biliary Sciences, New Delhi between June 2013 and June 2014 with the diagnosis of SBP (during hospitalization) and cirrhosis were reviewed. The study was approved by the Institutional Ethics Committee and the guidelines of Helsinki declaration were followed[10]. The Ethics Committee waived the requirement for the consent for data analysis.
The diagnosis of cirrhosis was based on clinical, laboratory and imaging findings. SBP was diagnosed by abdominal paracentesis in the presence of neutrophil count ≥ 250 cells/mm3 in the ascitic fluid and the absence of the secondary features suggestive of secondary bacterial peritonitis[11]. We also required a positive culture.
Patient charts were retrospectively reviewed. Data include patient demographics, etiology, severity of liver disease, laboratory values, co-existing medical diagnoses (diabetes mellitus, hepatocellular carcinoma), medication use, organ failure, ascitic fluid analysis results, duration of ICU stay, and patient outcome. In the case of culture-positive infections, all microorganisms and their antibiotic susceptibility patterns were recorded. Most patients admitted to the ICU were referred after a variable antibiotic exposure; prior systemic or non-absorbable antibiotic data could not be collected.
The laboratory parameters [bilirubin, creatinine levels and international normalized ratio (INR)] at admission to intensive care unit were used to calculate MELD-Na score using the UNOS Internet site[12]. As a protocol, all patients admitted/transferred to the ICU with ascites, underwent an ascitic fluid analysis within 24 h of admission, in the absence of severe coagulopathy. Ascitic fluid was sent for albumin and cell count with differential and cultured by inoculation of 10 mL of ascitic fluid in blood culture bottles. Paired blood culture samples were also collected at admission in all patients. Antibiotic choice varied from patient to patient, and no standard first-line drugs were used. The choice of antibiotic was decided based on previous antibiotic exposure of the patient before development of SBP, whether the patient was on quinolone prophylaxis, and physician discretion based on the perceived severity of patient illness. The antibiotic use was narrowed and modified as per the gram-stain and antibiotic sensitivity results. No patient underwent fluid restriction or hypertonic saline for management of dilution hyponatremia.
Renal dysfunction was defined by AKIN criteria[8] and managed by albumin infusions and intravenous terlipressin, with dose titrated as per response and tolerance. Intravenous albumin was used in all patients, with a minimal daily dose of 20 g and increased to up to 60 g/d[13], titrated by clinical monitoring and hourly urine output. We did not stratify renal dysfunction into hepato-renal syndrome (HRS), and non-HRS. However, any cause of secondary renal dysfunction was actively investigated, with urine sediment, 24-h urine protein excretion, and bedside-renal ultrasound. All patients were evaluated daily by a nephrologist. Hepatic encephalopathy was treated with oral and rectal lactulose and rifaximin. No patient received neomycin. We suspected secondary peritonitis in patients with inadequate response to therapy, severe abdominal tenderness or when multiple organisms were identified in the ascitic fluid. These patients underwent a non-contrast computed tomography (CT) scan of abdomen.
American College of Chest Physicians/Society of Critical Care Medicine consensus conference criteria were used to diagnose septic shock[14].
Patients with cirrhosis and ascites fluid polymorphonuclear cell (PMN) < 250 cells/mm3. Patients admitted from the community with SBP. Patients presented with ascites unrelated to cirrhosis. Patients with variceal haemorrhage advanced malignancy and human immunodeficiency virus.
Stata version 14 for Windows was used for analysis. All the variables were normally distributed with equal variance. The continuous variables were described as mean ± SD. The means of continuous variables were compared using student’s t test. Categorical variables were described as proportions. The means of categorical variables were compared with logistics regression. Multivariate logistics regression was employed to analyse statistically significant variables. Cox proportional hazard model was used to analyse the hazard rates of the predictors adjusted by age and gender. The predictive accuracy of the prognostic variables like MELD-Na, AKI and septic shock was measured using receiver operating characteristics (ROC) curves. The best cut-off point for MELD-Na was created using acceptable sensitivity and specificity in the ROC analysis to determine 50 d in-hospital mortality risk. For each predictor variable, sensitivity, specificity, positive predictive value (PPV), negative predictive values (NPV), positive likelihood ratio (+LR) and negative LR (-LR) were calculated to fit into the prognostic model. Two tailed P value < 0.05 was considered statistically significant. The power of the study was set at 80%. STROBE checklist for retrospective analysis was performed.
Total of 218 patients with decompensated cirrhosis with ascites and SBP were included in the study. Two hundred and eleven (97%) patients were diagnosed with SBP for the first time and only 7 patients (0.03%) had previous episodes (more than once). The 50 d in-hospital mortality rate was 43.11% (n = 94). Median survival duration for those who died was 9 d. In univariate analysis AKI, hepatic encephalopathy, septic shock, total leucocyte count, serum bilirubin, INR, aspartate transaminase (SGOT), and MELD-Na were significantly associated with in-hospital mortality in patients with SBP (Table 1).
| Variables | Overall (n = 218) | Survivors (n = 124) | Deaths (n = 94) | P value |
| Demographic data | ||||
| Age (yr) mean ± SD | 49.90 ± 12.52 | 49.86 ± 13.37 | 49.96 ± 11.37 | 0.950 |
| Male (%) | 177 (81.19) | 99 (79.84) | 78 (82.98) | 0.557 |
| Etiology of cirrhosis (%) | ||||
| Ethanol | 100 (45.87) | 48 (38.71) | 52 (55.32) | 0.689 |
| Crypto/NAFLD | 63 (28.90) | 38 (30.65) | 25 (26.60) | 0.104 |
| HCV | 23 (10.55) | 16 (12.905) | 7 (7.45) | 0.068 |
| Clinical data (%) | ||||
| Hepatocellular carcinoma | 17 (7.80) | 9 (7.26) | 8 (8.51) | 0.733 |
| Diabetes | 47 (21.56) | 27 (21.77) | 20 (21.28) | 0.929 |
| Acute kidney injury | 99 (45.41) | 35 (28.23) | 64 (68.09) | < 0.001 |
| Respiratory failure | 10 (4.59) | 6 (4.84) | 4 (4.26) | 0.978 |
| Hepatic encephalopathy | 109 (50.0) | 50 (40.32) | 59 (62.77) | 0.001 |
| Septic shock | 28 (12.84) | 4 (3.23) | 24 (25.53) | < 0.001 |
| Positive culture | 48 (22.02) | 21 (16.94) | 27 (28.72) | 0.038 |
| Laboratory data1 (mean ± SD) | ||||
| Ascitic neutrophil count (cells/mm3) | 3346.07 ± 4700.60 | 3899.28 ± 5003.75 | 2616.30 ± 4182.81 | 0.040 |
| Hemoglobin (g/dL) | 9.42 ± 1.88 | 9.58 ± 1.77 | 9.21 ± 2.01 | 0.154 |
| Platelet count (mmol/L) | 128.24 ± 102.11 | 138.43 ± 111.25 | 115.03 ± 87.69 | 0.095 |
| Leucocyte count (103/μL) | 13.30 ± 9.35 | 11.86 ± 8.65 | 15.17 ± 9.92 | 0.009 |
| Sodium (mEq/L) | 132.14 ± 7.69 | 132.50 ± 6.54 | 131.67 ± 9.01 | 0.454 |
| Billirubin (mg/dL) | 8.17 ± 8.81 | 5.85 ± 6.27 | 11.24 ± 10.61 | < 0.001 |
| Albumin (g/dL) | 2.32 ± 0.50 | 2.35 ± 0.48 | 2.28 ± 0.52 | 0.250 |
| INR | 2.31 ± 1.11 | 2.09 ± 1.08 | 2.59 ± 1.08 | 0.001 |
| AST (U/L) | 59.66 ± 109.81 | 79.23 ± 98.71 | 171.3 ± 321.94 | 0.003 |
| ALT (U/L) | 59.66 ± 109.81 | 46.49 ± 72.93 | 77.04 ± 143.41 | 0.041 |
| Urea (mg/dL) | 70.31 ± 52.42 | 62.24 ± 48.23 | 80.94 ± 55.98 | 0.008 |
| Creatinine (mg/dL) | 1.67 ± 1.29 | 1.58 ± 1.39 | 1.80 ± 1.15 | 0.217 |
| Scores (mean ± SD) | ||||
| CTP (B/C) | 10.72 ± 1.82 | 10.50 ± 1.95 | 11.02 ± 1.60 | 0.034 |
| MELD | 24.79 ± 8.28 | 22.20 ± 7.59 | 28.20 ± 7.94 | < 0.001 |
| MELD-Na | 27.53 ± 7.57 | 25.21 ± 7.44 | 30.59 ± 6.62 | < 0.001 |
The baseline characteristics of the demographics, etiology, clinical and laboratory data is shown in Table 1. Mean age was 49.90 ± 12.52 years and the male was predominant (83%). Most common etiology of liver cirrhosis was ethanol-induced (45.87%) followed by crypto/non-alcoholic fatty liver disease-NAFLD (28.9%). Hepatitis C virus related cirrhosis constitute only 11% in this study. A total of 109 subjects (50.0%) had hepatic encephalopathy with 59 deaths (62.77%), P = 0.001. Overall, 99 patients (45.11%) had AKI in hospitalized patients out of which 64 died (68.09%), P < 0.001. Compared with survivors the deceased had a higher proportion of septic shock (25.53% vs 3.23%), P < 0.001. Total leukocyte counts, bilirubin, INR, SGOT were significantly higher in the patients who died compared to the survivors. Mean MELD-Na score was higher among the deaths comparing to the survivors (30.59 ± 6.62 vs 25.21 ± 7.44) with statistical significance (P < 0.001). Child-Turcotte-Pugh (CTP) (B/C) score was not different among the groups. The mean CTP scores were high with mean 10.72 (SD: 1.82).
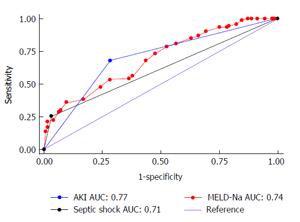
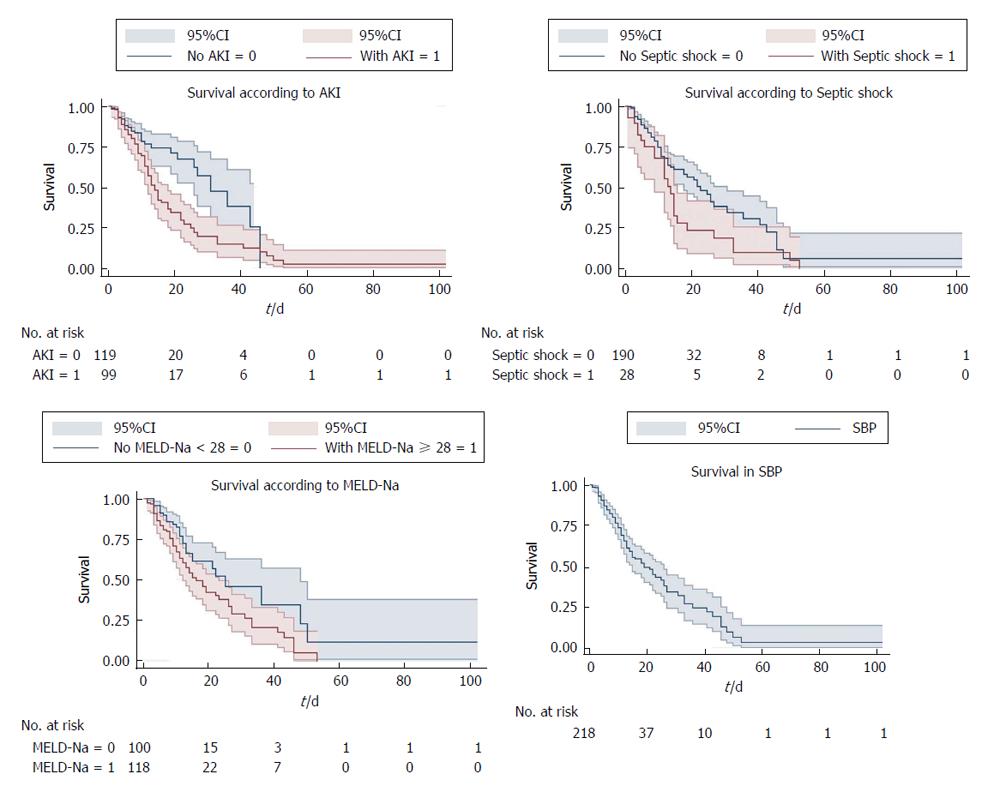
On multivariate regression analysis, AKI (P = 0.001), septic shock (P = 0.029), MELD-Na (P < 0.001) were found to be independent predictors of 50 d in-hospital mortality in patients with SBP (Table 2). Cox proportional hazard model showed the hazard ratio (HR) of AKI was 2.16 (95%CI: 1.36-3.42), septic shock (HR = 1.73, 95%CI: 1.05-2.83) and MELD-Na (HR = 1.1, 95%CI: 1.02-1.21). ROC curve for AKI, septic shock and MELD-Na had better prognostic accuracy for 50 d in-hospital mortality in patients with SBP (Figure 1). AKI had highest area under the curve (AUC) 0.77 (95%CI: 0.71-0.83), followed by MELD-Na (AUC: 0.74, 95%CI: 0.69-0.79), septic shock (AUC: 0.71, 95%CI: 0.65-0.77). Table 3 reports the sensitivity, specificity, PPV, NPV, +LR and -LR for these predictors. The cut off for MELD-Na derived from the ROC with the best ability to predict 50 d in-hospital mortality in decompensated cirrhotic patient with SBP was 28, with sensitivity 92.9%, specificity 60.3%, and NPV of 97.9%. The Kaplan-Meier survival analysis was plotted for the 50-d survival in SBP patients along with individual prognostic variables like AKI, MELD-Na, and septic shock (Figure 2).
| Variables | 1HR (95%CI) | P value |
| AKI | 2.16 (1.36-3.42) | 0.001 |
| Septic shock | 1.73 (1.05-2.83) | 0.029 |
| MELD-Na | 1.06 (1.02-1.09) | < 0.001 |
The prevalence of SBP in outpatients has been reported to be 1.5%-3.5%[15]. Among in-patients the prevalence is around 10%[15]. Half of the episodes of SBP are acquired during hospitalization. In the present observational study SBP related 50 d in-hospital mortality in decompensated cirrhosis was 43%. Of total 94 cases, 93 patients with SBP (99%) died on or before 50th d of hospitalisation.
This study assessed different prognostic factors which can be used to predict mortality in hospitalized patient with SBP and corroborates that hepatic encephalopathy, total leukocyte count, serum bilirubin, SGOT, INR and child pugh score significantly associated with mortality[5,7,16]. The MELD score shows promise as a means for risk - stratifying patients with SBP including those waiting for liver transplantation[7]. Certain limitations of MELD model[17] prompted us to include MELD-Na as hyponatremia is a well-known predictor of death in cirrhotic patients. Isolated creatinine is inaccurate measurement of renal failure in decompensated liver cirrhosis due to significant reduction in creatinine production in liver and muscle wasting[18]. We found AKI, MELD-Na and septic shock to be the important predictors of mortality. We did not incorporate other independent variables like total leukocyte count, serum bilirubin, INR since these were the components in the present predictive model like MELD-Na and septic shock.
In this study AKI has the single best predictive ability (AUC: 0.77) followed by MELD-Na (AUC: 0.74) and septic shock (AUC: 0.71). In addition, we identified MELD-Na cut-off 28 with sensitivity 92.9% and NPV of 97.9%. The hazard ratio of mortality for patients with AKI was significantly higher 2.16 (95%CI: 1.36-3.42) compared to septic shock (HR = 1.73, 95%CI: 1.05-2.83) and MELD-Na (HR = 1.06, 95%CI: 1.02-1.09). Kaplan-Meier survival analysis showed AKI, MELD-Na, and septic shock as predictors for the 50 d in-hospital mortality in decompensated patients with SBP. It can help in the further improvement of the quality of care of hospitalized SBP patients with reduction of their short-term mortality. The cut-off for MELD-Na can be applied to prioritize high-risk patients upon hospital admission who would benefit by expectant management.
Diagnosis of SBP is based on the demonstration of an absolute number of PMNs in ascitic fluid equal to or greater than 250/mm3. However, the best specificity for diagnosis has been reported with a cut-off of 500 PMN/mm3. It is unclear whether a positive culture in the absence of elevated ascitic fluid PMN count (bacteriascites), requires antibiotic therapy. In these cases, some guidelines recommend antibiotic treatment only if the patient shows signs of infection[18]. Ascitic fluid culture is positive in 40% of all cases. The most common isolates include GNB, usually Escherichia coli and Gram-positive cocci (mainly Streptococcus species and Enterococci)[3]. Gram negative organism infections predominate in community acquired and gram-positive organisms in nosocomial infections[3]. Recommended first-line antibiotics for treatment of SBP include third generation cephalosporins (mainly Cefotaxime), Amoxicillin-Clavulanic acid, ciprofloxacin, and ofloxacin[19], with an expected resolution rate of over 90%.These guidelines from the western medicine acknowledge the increasing problem of antibiotic resistance[20] and recommend coverage for resistant organisms if there is no evidence of infection resolution at repeat ascitic fluid analysis at 48 h. Resistant infections are usually caused by Enterococcus faecium and extended-spectrum β-lactamase-producing Enterobacteriaceae, which are resistant to the current recommended empirical antibiotic therapy[21]. These findings led to the suggestion that nosocomial SBP should be treated with carbapenems or with tigecycline[22]. We included only patients with hospital-acquired SBP because most of the present ICU admissions include transferred patients already hospitalised, with a variable but consistent antibiotic exposure. Only a minority of our patients are admitted directly from the community, and usually to the wards and not to the ICU. These patients would be expected to have a higher prevalence of resistant infections. A hospital-acquired infection was an independent predictor of death, likely due to a higher rate of multidrug resistance (resistance to third-generation cephalosporin)[23].
This study has certain strengths and limitations. The results clearly show AKI has greater predictive ability than septic shock and MELD-Na as far as 50 d in-hospital mortality in SBP patient is concerned. This study did not account for the stages of ascites. We didn’t stratify our patients according to different stages of AKI as per AKIN criteria. We didn’t consider HRS into account in this study. We didn’t evaluate the antibiotic resistance in SBP patients who are culture positive at the baseline. We included only nosocomial acquired SBP. Most of our patients presented with advanced decompensated liver cirrhosis at the time of SBP diagnosis. The advanced liver cirrhosis was assessed by lower serum albumin, high serum bilirubin and INR values. This study is a single centre study, these findings needed to be supplemented by multicentre prospective studies.
Spontaneous bacterial peritonitis (SBP) is associated with poor prognosis especially in-hospital patients. The mortality rate ranges from 20%-40%. The model for end-stage liver disease (MELD) has been suggested as a predictor of the in-hospital mortality in patients with SBP. The authors’ goal is to identify other prognostic factors for mortality in decompensated cirrhotic patients with SBP and to evaluate the predictive power of acute kidney injury (AKI), MELD-sodium (MELD-Na) and septic shock to predict mortality.
The prognostic factors for mortality with SBP patients in liver cirrhosis are important in determining the management. MELD has been considered as an important predictive factor. But it’s not clear about role of other prognostic factors.
In this study, 50 d in-hospital mortality rate attributable to SBP is 43.11%. receiver operating characteristic (ROC) curve, Kaplan Meier survival analysis was useful tool in predicting 50 d in-hospital mortality in SBP with liver cirrhosis. Multivariate cox proportional regression analysis showed AKI [hazrd ratio (HR) = 2.16, 95%CI: 1.36-3.42, P = 0.001] septic shock (HR = 1.73, 95%CI: 1.05-2.83, P = 0.029) MELD-Na (HR = 1.06, 95%CI: 1.02-1.09, P≤ 0.001) were significantly associated with 50 d in-hospital mortality. The prognostic accuracy for AKI, MELD-Na and septic shock was 77%, 74% and 71% respectively.
Liver transplant is potentially only curative therapeutic option with long term result in patients with decompensated cirrhosis and SBP. The cost of liver transplant and the shortages of the liver donor is a point of concern. The findings of this study can be used as a strategic approach in advanced liver cirrhosis patients on hospital admission that would benefit from intensive management where liver transplant is not a plausible option. It can help in the further improvement of the quality of care of hospitalized SBP patients with reduction of their short-term mortality. The cut-off for MELD-Na can be used to stratify high-risk patients on hospital admission who would benefit by intensive management.
The ROC analysis is a graphical plot in statistical methods to create a cut off value for the predictors. The graph is plotted with true positive value against false positive value. The accuracy of cut off value is interpenetrated by the area under curve (AUC) in ROC curve. AUC = 1 is gold standard, 0.9-1 = excellent, 0.8-0.9 = good, 0.7-0.8 = fair, 0.6-0.7 = poor, ≤ 0.5 = fail. Kaplan-Meier survival curve is a time to event analysis of series of events over a period of time recorded in horizontal and declining horizontal steps. When a person withdraws from the study (censored), lost follow up, or died there will be a sudden drop in the curve. HR: It’s a ratio of hazard rates. It is the probability of an event in the study group or population at a particular time. HRs are used in time to event analysis.
This is a retrospective study to analyze predictors of 50 d in-hospital mortality in decompensated cirrhosis patients with spontaneous bacterial peritonitis. The authors review the medical records of 218 adults admitted with SBP in period of one year, to identify factors related to mortality. The article is very well described; it was properly planned and conducted.
P- Reviewer: Haddad L, Tovo CV S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
| 1. | Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 650] [Cited by in F6Publishing: 589] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 2. | Runyon BA. Ascites and spontaneous bacterial peritonitis. Sleisenger and Fordran’s Gastrointestinal and Liver Disease, 8th ed. Philadelphia, PA: Saunders 2006; 1935-1964. [Cited in This Article: ] |
| 3. | Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut. 2012;61:297-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 4. | Evans LT, Kim WR, Poterucha JJ, Kamath PS. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology. 2003;37:897-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Thuluvath PJ, Morss S, Thompson R. Spontaneous bacterial peritonitis--in-hospital mortality, predictors of survival, and health care costs from 1988 to 1998. Am J Gastroenterol. 2001;96:1232-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Zhang QB, Chen YT, Lian GD, Qian CC, Chen SJ, Huang KH. A combination of models for end-stage liver disease and cirrhosis-related complications to predict the prognosis of liver cirrhosis. Clin Res Hepatol Gastroenterol. 2012;36:583-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Nobre SR, Cabral JE, Gomes JJ, Leitão MC. In-hospital mortality in spontaneous bacterial peritonitis: a new predictive model. Eur J Gastroenterol Hepatol. 2008;20:1176-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | de Carvalho JR, Villela-Nogueira CA, Luiz RR, Guzzo PL, da Silva Rosa JM, Rocha E, Moraes Coelho HS, de Mello Perez R. Acute kidney injury network criteria as a predictor of hospital mortality in cirrhotic patients with ascites. J Clin Gastroenterol. 2012;46:e21-e26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Eduardo RP, Margarida FS. Hepatorenal syndrome, septic shock and renal failure as mortality predictors in patients with spontaneous bacterial peritonitis. GE J Port Gastroenterol. 2012;19:278-283. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. Available from: http://www.wma.net/en/30publications/10policies/b3/. [Cited in This Article: ] |
| 11. | Guarner C, Soriano G. Spontaneous bacterial peritonitis. Semin Liver Dis. 1997;17:203-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 118] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | United Network for Organ Sharing (UNOS). Available from: http: //www.unos.org/. [Cited in This Article: ] |
| 13. | Alves de Mattos A. Current indications for the use of albumin in the treatment of cirrhosis. Ann Hepatol. 2011;10 Suppl 1:S15-S20. [PubMed] [Cited in This Article: ] |
| 14. | Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644-1655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6211] [Cited by in F6Publishing: 6303] [Article Influence: 197.0] [Reference Citation Analysis (0)] |
| 15. | Nousbaum JB, Cadranel JF, Nahon P, Khac EN, Moreau R, Thévenot T, Silvain C, Bureau C, Nouel O, Pilette C, Paupard T, Vanbiervliet G, Oberti F, Davion T, Jouannaud V, Roche B, Bernard PH, Beaulieu S, Danne O, Thabut D, Chagneau-Derrode C, de Lédinghen V, Mathurin P, Pauwels A, Bronowicki JP, Habersetzer F, Abergel A, Audigier JC, Sapey T, Grangé JD, Tran A. Diagnostic accuracy of the Multistix 8 SG reagent strip in diagnosis of spontaneous bacterial peritonitis. Hepatology. 2007;45:1275-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Terg R, Gadano A, Cartier M, Casciato P, Lucero R, Muñoz A, Romero G, Levi D, Terg G, Miguez C. Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study. Liver Int. 2009;29:415-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Gotthardt D, Weiss KH, Baumgärtner M, Zahn A, Stremmel W, Schmidt J, Bruckner T, Sauer P. Limitations of the MELD score in predicting mortality or need for removal from waiting list in patients awaiting liver transplantation. BMC Gastroenterol. 2009;9:72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 628] [Cited by in F6Publishing: 588] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 19. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1125] [Cited by in F6Publishing: 1070] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 20. | Cheong HS, Kang CI, Lee JA, Moon SY, Joung MK, Chung DR, Koh KC, Lee NY, Song JH, Peck KR. Clinical significance and outcome of nosocomial acquisition of spontaneous bacterial peritonitis in patients with liver cirrhosis. Clin Infect Dis. 2009;48:1230-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Ariza X, Castellote J, Lora-Tamayo J, Girbau A, Salord S, Rota R, Ariza J, Xiol X. Risk factors for resistance to ceftriaxone and its impact on mortality in community, healthcare and nosocomial spontaneous bacterial peritonitis. J Hepatol. 2012;56:825-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551-1561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 392] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 23. | Umgelter A, Reindl W, Miedaner M, Schmid RM, Huber W. Failure of current antibiotic first-line regimens and mortality in hospitalized patients with spontaneous bacterial peritonitis. Infection. 2009;37:2-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |