Published online Feb 27, 2015. doi: 10.4254/wjh.v7.i2.165
Peer-review started: July 16, 2014
First decision: August 28, 2014
Revised: September 30, 2014
Accepted: November 17, 2014
Article in press: November 19, 2014
Published online: February 27, 2015
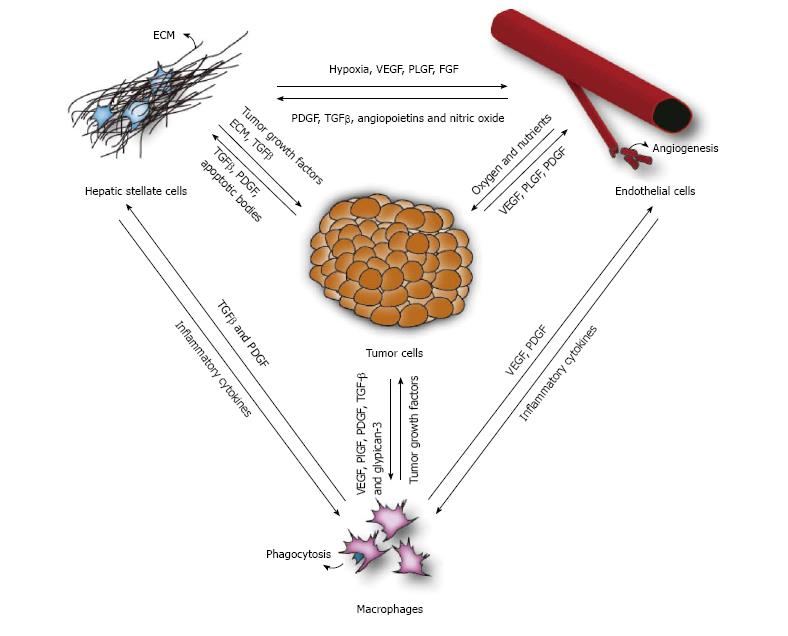
Hepatocellular carcinoma (HCC) is one of the most common and deadly cancers worldwide. In ninety percent of the cases it develops as a result of chronic liver damage and it is thus a typical inflammation-related cancer characterized by the close relation between the tumor microenvironment and tumor cells. The stromal environment consists out of several cell types, including hepatic stellate cells, macrophages and endothelial cells. They are not just active bystanders in the pathogenesis of HCC, but play an important and active role in tumor initiation, progression and metastasis. Furthermore, the tumor itself influences these cells to create a background that is beneficial for sustaining tumor growth. One of the key players is the hepatic stellate cell, which is activated during liver damage and differentiates towards a myofibroblast-like cell. Activated stellate cells are responsible for the deposition of extracellular matrix, increase the production of angiogenic factors and stimulate the recruitment of macrophages. The increase of angiogenic factors (which are secreted by macrophages, tumor cells and activated stellate cells) will induce the formation of new blood vessels, thereby supplying the tumor with more oxygen and nutrients, thus supporting tumor growth and offering a passageway in the circulatory system. In addition, the secretion of chemokines by the tumor cells leads to the recruitment of tumor associated macrophages. These tumor associated macrophages are key actors of cancer-related inflammation, being the main type of inflammatory cells infiltrating the tumor environment and exerting a tumor promoting effect by secreting growth factors, stimulating angiogenesis and influencing the activation of stellate cells. This complex interplay between the several cell types involved in liver cancer emphasizes the need for targeting the tumor stroma in HCC patients.
Core tip: Hepatocellular carcinoma is a primary liver tumor that usually develops in a background of chronic liver disease and fibrosis. It is the underlying chronic inflammation that creates an environment that not only causes but also enhances the formation and growth of tumors. The stromal compartment-including hepatic stellate cells, macrophages and endothelial cells-actively contribute to tumorigenesis, while the tumor itself influences these cells to create a background that is beneficial for tumor growth. This review focuses on the interplay between stroma and tumor cells, as well as therapeutic strategies that aim to target these complex interactions.
- Citation: Heindryckx F, Gerwins P. Targeting the tumor stroma in hepatocellular carcinoma. World J Hepatol 2015; 7(2): 165-176
- URL: https://www.wjgnet.com/1948-5182/full/v7/i2/165.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i2.165
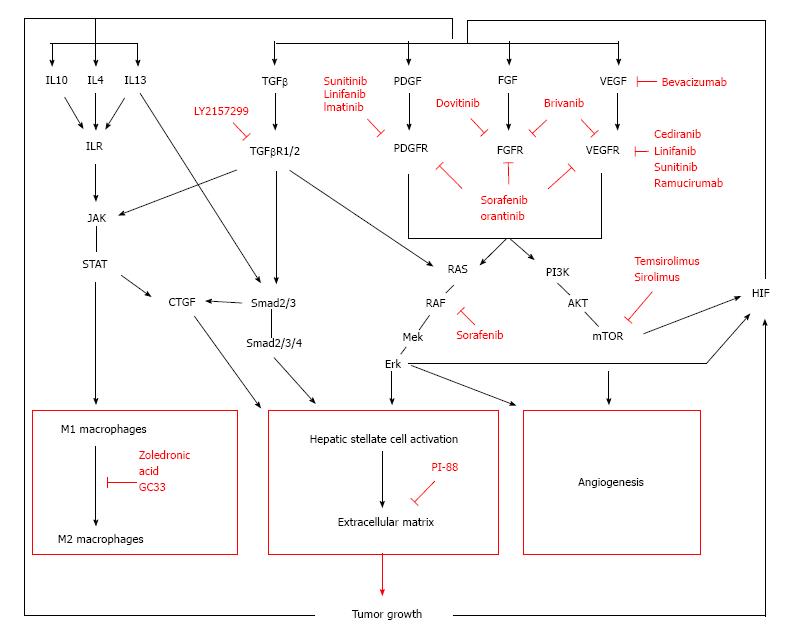
Hepatocellular carcinoma (HCC) is a primary liver tumor that usually develops in a background of chronic liver disease. It is the underlying chronic inflammation that creates an environment that not only causes but also enhances the formation and growth of tumors. Firstly, the continuous state of inflammation as a result of sustained liver damage can lead to hepatocyte cell death as well as compensatory proliferation, which can generate an accumulation of genomic lesions in hepatocytes. Secondly, the initiated cells are surrounded by an inflammatory niche that facilitates their progression towards malignant tumors. For instance, the fibrotic liver is characterized by an increased formation of blood vessels[1], which will benefit tumor cells for their blood supply as well as facilitating metastasis[2]. In addition, several factors produced by macrophages and activated stellate cells are known to directly stimulate and enhance tumor growth. Once the cancer has been established, the microenvironment continues to regulate the tumor behavior, influencing the development, progression and even response to therapy. All players within the tumor stroma strongly interact with each other, creating an environment that supports tumor growth (Figure 1). It is therefore not unlikely that future therapies will more and more focus on targeting these complex interactions in the tumor stroma (Figure 2). Ongoing clinical trials are listed in Table 1.
| Drug | Targets | Trial | Phase | Status | Ref. | |
| Tyrosine kinase inhibitors | Sorafenib | PDGFR | NCT00105443 | III | Completed1 | [90] |
| VEGFR | ||||||
| RAF/MEK/ERK | ||||||
| Orantinib | PDGFR, FGFR | NCT02178358 | I/II | Completed | [91] | |
| VEGFR | ||||||
| Sunitinib | VEGFR | NCT00699374 | III | Terminated | [71] | |
| PDGFR | NCT00514228 | II | Completed | [70] | ||
| RET | NCT00361309 | II | Completed | [69] | ||
| CSF | NCT00428220 | N/A | Ongoing | |||
| Linifanib | VEGF, PDGF, PDGFR-β, KDR | NCT01009593 | III | Terminated | ||
| CSF | NCT00517920 | II | Completed | [68] | ||
| Brivanib | VEGFR | NCT00858871 | III | Completed | [59] | |
| FGFR | NCT00908752 | III | Ongoing | |||
| NCT00825955 | III | Ongoing | ||||
| NCT01108705 | III | Terminated | ||||
| NCT00355238 | II | Completed | [92] | |||
| NCT00437424 | I | Completed | [93] | |||
| Cediranib | VEGFR | NCT00238394 | II | |||
| NCT00427973 | II | Terminated | [54] | |||
| Dovitinib | VEGFR, PDGFR, FGFR | NCT01232296 | II | Ongoing | ||
| Antibodies | Bevacizumab | VEGF | NCT00335829 | II | Completed | [94] |
| NCT00162669 | II | Completed | [50] | |||
| NCT00605722 | II | Completed | [51] | |||
| NCT00049322 | II | Completed | [52] | |||
| NCT00280007 | II | Terminated | ||||
| NCT01180959 | II | Ongoing | ||||
| Ramucirumab | VEGFR | NCT00627042 | II | Completed | [53] | |
| NCT01140347 | III | Ongoing | ||||
| GC33 | Glypican-3 | NCT01507168 | II | Completed | ||
| NCT00746317 | I | Completed | [84] | |||
| NCT00976170 | I | Ongoing | ||||
| Other kinase inhibitors | Temsirolimus | mTOR | NCT01008917 | I | Ongoing | |
| NCT01687673 | II | Recruiting | ||||
| Everolimus | mTOR | NCT01035229 | III | Completed | [95] | |
| NCT01488487 | II | Ongoing | ||||
| NCT00516165 | I/II | Completed | [66] | |||
| NCT00828594 | I | Terminated | ||||
| LY2157299 | TGF-βR1 | NCT01246986 | II | Recruiting | ||
| NCT02178358 | II | Recruiting | ||||
| PI-88 | Heparanase | NCT00568308 | III | Terminated | ||
| NCT01402908 | III | Ongoing | ||||
| NCT00247728 | II | Completed | [30] | |||
| Zoledronic acid | Macrophages | NCT01259193 | II | Ongoing |
One major player in the formation of the perfect tumor environment is the activated hepatic stellate cell (HSC)[3,4]. During liver injury, the stellate cells undergo a transformation from quiescent cells that serve as the liver’s resident vitamin-A storing cells, towards “activated” myofibroblast-like cells. These activated HSCs are characterized by increased proliferation and contractility, altered matrix protease activity and the secretion of extracellular matrix (ECM) proteins, as well as tumor growth factors and pro-angiogenic factors.
Several studies have shown that co-culturing HSC with different HCC cell lines induces phenotypic changes in the behavior of the tumor cells[5,6]. In vitro studies show that HSCs can directly influence the tumor cells (through the secretion of growth factors[7], matrix proteases[8] and/or ECM proteins[9]) and there is also evidence from in vivo studies that activated HSCs can create an immunosuppressive environment that promotes HCC growth[10,11]. The interaction between the tumor cells and HSCs is bidirectional, thereby allowing the tumor to alter the stellate cells (and the overall stromal environment) towards a more pro-tumoral phenotype[8]. Consistent with these findings, several in vivo studies have shown that inducing stellate cell activation increases liver fibrosis and hepatocarcinogenesis[12-15].
One of the key factors in this HSC-HCC cross talk is transforming growth factor (TGF)-β[14,16,17]. Activated HSC are the main source of TGF-β, however most liver cells (including malignant hepatocytes) have the ability to produce TGF-β as well. The TGF-β signaling pathway consists of three distinct ligands, TGF-β1, TGF-β2, and TGF-β3 which all bind to a specific receptor by first engaging with the TGF-βR1, which then heterodimerizes with the TGF-βR2. This causes the phosphorylation of Smad2 and 3, initiating an activation cascade leading to the induction several nuclear transduction proteins. Alternative pathway activation is possible, including the activation of AKT and other intracellular activation proteins. Interestingly, Smad7 antagonizes TGF-β mediated activation of hepatic stellate cells and protects against liver damage[18]. TGF-β signaling promotes HCC by several distinct mechanisms (reviewed more in detail by Dooley et al[17]): firstly, through functioning as a growth factor, by which it can act oncogenic or as tumor suppressor depending on the temporal and spatial availability of TGF-β in tumor and stromal cells[19,20]. And secondly, by transforming HSC to activated myofibroblasts. Interestingly, Inhibitors of TGF-β signaling have been shown to block HCC in different experimental models[21], leading to the clinical investigation of the TGF-β inhibitor LY2157299 (NCT01246986 and NCT02178358). LY2157299 is a small molecule kinase inhibitor that binds to TGF-βR1 and hence inhibits TGF-β signaling.
The connective tissue growth factor (CTGF) is an extracellular matrix-associated heparin binding protein that is overexpressed in fibrotic lesions, and the overexpression correlates with the severity of fibrosis and can be linked to malignant transformation in patients with chronic hepatitis B[22]. CTGF is a downstream mediator of some TGF-β effects and it is induced by TGF-β in a SMAD2/3 and stat3 dependent way. Furthermore, IL-13 is able to induce CTGF expression in HSCs by activating TGF-β-independent Smad signaling via the Erk-MAPK pathway instead of the canonical JAK/Stat6 pathway[23]. CTGF expression in HSC leads to increased migration, proliferation, and collagen expression of these cells. In addition, studies have shown that TGF-β can elicit a direct effect on hepatocytes via CTGF, thus making it an interesting therapeutic target for multiple cell types involved in the fibrogenesis[18]. CTGF blocking antibodies have been tested in patients with idiopathic pulmonary fibrosis (NCT00074698) and animal studies have shown that CTGF-inhibition prevents liver fibrosis in rats[24]. However, no clinical trials on the effect on liver fibrosis have been done.
Another driver of HSC activation are members of the platelet derived growth factor (PDGF) family. PDGFs are potent mitogens for mesenchymal cells and work synergistically with TGF to activate stellate cells. Specific hepatic over-expression of PDGF-C leads to an increase in fibrosis and enhances hepatocarcinogenesis[12,15]. PDGF-B is also involved in different stages of liver cancer development and is an essential regulator in the development of liver fibrosis[25]. Hepatic overexpression of PDGF-B accelerates liver cancer, possibly by up regulating TGF-β receptor and by increasing expression of β-catenin as well as VEGF, CD31 and FGF. Several protein tyrosine kinase inhibitors-such as sorafenib, orantinib, sunitinib and SU6668-target PDGFR amongst other targets including VEGFR and FGFR. The protein tyrosine kinase inhibitor imatinib reduces stromal cell proliferation in this mouse model, which successfully inhibits tumor progression[13]. Imatinib is currently used to treat gastrointestinal stromal tumors[26] and could possibly benefit HCC patients.
The deposition of ECM proteins is one of the most characteristic hallmarks of the activated stellate cell. Several of the ECM components such as proteoglycans, laminins, collagens, and fibronectin interact directly and indirectly with HCC cells and the different stroma cell types. This not only changes the tumor phenotype, but also prepares a microenvironment that facilitates tumor growth. Since the ECM acts as a reservoir for growth factors and cytokines, it can rapidly release them to support the tumor’s needs.
Heparan sulfate (HS) proteoglycans (PG) are expressed in the ECM and composed of a protein core to which HS is covalently attached as side chains. They maintain the structural framework of the tissue, store growth factors within the ECM or function as co-receptors. Desulfation of these co-receptor-PG’s can abrogate growth factor signaling and inhibit tumor growth[27,28]. Heparanase cleaves the HS side chains of HSPG, leading to the release of HS-bound proteins, such as growth factors. PI-88 is a heparin sulfate mimic that specifically targets heparanase in cancer, thus preventing the release of growth factors that otherwise would contribute to tumor growth, angiogenesis and metastasis[29]. The safety and efficiency of PI-88 as an adjuvant therapy for post-operative HCC has been shown in a phase II trial[30] and a recent follow up study revealed significant clinical benefits for patients with HCC[31]. Phase III trials are currently ongoing (NCT01402908).
Another important glycoprotein is laminin-5. Laminin-5 is a member of the laminin family, which has been widely reported to be involved in the malignant phenotype of several cancers, including HCC[32]. Laminin-5 is expressed higher in metastatic HCC and has been shown to stimulate HCC cell migration[9,32].
Collagen is the major insoluble fibrous protein in the extracellular matrix. Besides its function as a supportive scaffold, collagens can also provoke a cellular response through the integrin family of transmembrane receptors. Several collagen types have been implicated in tumor growth and angiogenesis in different tumors[33-35] and a recent study has shown that collagen matrix protects malignant hepatocytes from apoptosis[36]. Antibodies targeting cleaved collagen epitopes have been clinically tested and show promising results in patients with solid tumors[37,38].
This deposition of ECM leads to an increase in liver stiffness, an important hallmark of the cirrhotic liver, which is also used as a diagnostic tool for patients with CLD[39]. This change in the mechanical properties of the tumor’s surrounding has been associated with a higher risk of developing HCC[40]. In addition, the increase of ECM and the capillarization of hepatic sinusoids cause a vascular resistance that leads to hypoxia, stimulating the production of pro-angiogenic factors and subsequently inducing angiogenesis[1,41-43]. The activated HSCs also produce angiogenic growth factors, thus enhancing neo-angiogenesis[8]. This increased vasculature will allow small HCC lesions to progress and eventually metastasize.
The prolonged fibrogenic process leads to an abnormal angioarchitecture distinctive for cirrhosis. Anatomical changes in the cirrhotic liver, such as fibrotic scar tissue compressing portal and central venules are responsible for an increased intrahepatic vascular resistance. In addition, the formation of fibrotic septa, as well as sinusoidal capillarisation, results in an increased resistance to blood flow and oxygen delivery. This causes hypoxia and the transcription of hypoxia-sensitive pro-angiogenic genes, thus stimulating the formation of new vessels. These new vessels can contribute to the inflammatory response by expressing chemokines and adhesion molecules, thus promoting the recruitment of inflammatory cells, such as macrophages. In addition, hepatic stellate cells are recruited to the angiogenic areas (via a number of signaling pathways, including PDGF, TGF-β, angiopoetins and nitric oxide) to contribute in vascular remodeling and stabilization[44]. Therefore, angiogenesis may contribute to the progression of liver cirrhosis and stimulate the growth of small dysplastic lesions to advanced solid tumors.
HCC is solid tumor that rapidly outgrows its blood supply and therefore stimulates the formation of new blood vessels to fulfill its high needs in oxygen and nutrients. The malignant hepatocytes, as well as other actors in the microenvironment such as activated stellate cells and macrophages, secrete a number of angiogenic growth factors[1]. This induces an “angiogenic switch”, which activates endothelial cells and basement membranes to remodel existing vessels, and form new vessels. These new vessels allow the tumor to rapidly expand and offer a passage in the circulatory system, thus facilitating metastasis. Therefore, targeting angiogenesis has become a common cancer therapy to treat solid tumors.
The vascular endothelial growth factor A (VEGF) is one of the key factors regulating angiogenesis. It is secreted by tumor cells, macrophages and stellate cells. VEGF binds to its receptors (VEGFR1 and VEGFR2) on the present endothelial cells, simulating endothelial cell proliferation and migration into the tumor, which results in vascular sprouting. Elevated VEGF levels are associated with tumor vascularity, metastasis, chemoresistance and poor prognosis[45-47].
Significant progress on the treatment of advanced HCC has been made possible by sorafenib. Sorafenib is a small molecular inhibitor targeting several tyrosine protein kinases in the Raf/MEK/ERK-pathway (anti-proliferative effect); and PDGF, VEGFR1 and VEGFR2 (anti-angiogenic effect). Sorafenib has become the standard-of-care for patients with advanced HCC and for those progressing after loco-regional therapies[48]. The success of sorafenib has opened the door for several anti-angiogenic agents to enter clinical studies on HCC[49]. At the moment, several multikinase inhibitors are being tested in clinical trials, including sunitinib, brivanib, linifanib, cediranib, pazopanib, lenvatinib and axitinib, as well as blocking-antibodies targeting angiogenic pathways.
Bevacizumab, a humanized monoclonal antibody that targets VEGF, has been approved for the treatment of various solid tumors and is currently being investigated as a treatment for HCC. Several phase II trials have been completed and show that bevacizumab is well tolerated in HCC-patients, and could be a promising therapy as a single-agent[50], in combination with erlotinib[51] or after loco-regional therapies[52]. Ramucirumab is a monoclonal antibody targeting VEGFR2 which has been tested as a first line treatment (NCT00627042) for HCC-patients with promising results[53] and is currently being investigated as second line treatment after sorafenib (NCT01140347)[53]. Cediranib is a tyrosine kinase inhibitor that targets all VEGF receptors, which has been tested in two clinical trials (NCT00427973 and NCT00238394). Despite some anti-tumor effects, the high toxicity of cediranib makes it an unsuitable drug for HCC-patients HCC[54,55].
However, targeting VEGF has been shown to induce therapy escape mechanisms and many patients treated with VEGF-inhibitors or with sorafenib obtain a secondary resistance to therapy. Alternative angiogenic factors, such as the placental growth factor (PlGF), PDGF and fibroblast growth factor (FGF) have been implicated in this acquired tumor resistance and combination therapies could open the door for sustained treatment response[56]. Additionally, combining sorafenib with conventional chemotherapy could improve outcome and is currently tested in several phase III trials (NCT01015833, NCT01214343)[57].
Brivanib and dovitinib are tyrosine kinase inhibitors of VEGF and fibroblast growth factor (FGF) signaling pathways, hence anticipating FGF-mediated resistance to anti-VEGF therapy[58]. Brivanib has been or is being investigated in several phase III trials, including first-line treatment with brivanib vs sorafenib (NCT00858871)[59], second-line treatment with brivanib after progression on sorafenib treatment (NCT01108705), second-line treatment with brivanib after sorafenib (NCT00825955) and transarterial chemoembolization in combination with brivanib (NCT00908752). However, results from the study testing brivanib and sorafenib as first-line therapy in patients with HCC indicate there are no benefits of using brivanib over sorafenib[59] and study NCT01108705-testing brivanib after sorfanib treatment-has been terminated before completing the trial. Dovitinib trials are still ongoing (NCT01232296).
A drawback of anti-angiogenic therapies is that they aim to deprive the tumor from oxygen, leading to a hypoxic environment that stimulates cancer cells towards a more aggressive phenotype[60]. Therefore, long-term administration of anti-angiogenic treatment could trigger escape mechanisms and lead to increased metastasis[61,62].
An interesting way to indirectly target VEGF signaling and the HIF-pathway, is through inhibitors of the mammalian target of rapamycin (mTOR) pathway. mTOR signaling increases VEGF expression by up-regulating hypoxia inducible factor 1α[63]. Furthermore, mTOR-inhibitors can directly influence tumor growth by inhibiting the expression of anti-apoptotic proteins and by inducing autophagy[64]. Everolimus binds the cyclophilin FKBP-12, which binds the serine-threonine (ST) kinase mTOR when it is associated with raptor and mLST8 to form a complex (mTORC1), and subsequently inhibits downstream signaling, which involves cell cycle regulators and transcription factors such as HI. mTORC1 lies downstream of phosphatidylinositol 3′ kinase (PI3K), which is frequently activated in human cancers. Everolimus has been used in several clinical trials[65,66], but data from the latest phase III trial (NCT01035229) show no improval in overall survival[67]. Temsirolimus is a sirolimus ester, which binds the same receptors. Trials using temsirolimus are currently ongoing (NCT01008917 and NCT01687673).
The activated stellate cells also play a pivotal role in vascular remodeling, by creating a hypoxic environment, by producing angiogenic factors and also by migrating to angiogenic sites to contribute in the stabilization and maturation of (tumor) blood vessels. Current anti-angiogenic strategies for cancer have mostly focused on endothelial cells. However, combining drugs that target endothelial cells and stellate cells (or pericytes) could work synergistically as a therapy.
Several receptor tyrosine kinase inhibitors target VEGF and PDGF. Linifanib is a potent inhibitor of VEGF, PDGF, PDGFR-β, KDR and colony stimulating facto-1-receptor (CSF). A phase II trial (NCT00517920) showed initial benefits for linifanib in HCC patients[68], however, the subsequent phase III trial (NCT01009593) had to be terminated for unknown reasons. Sunitinib inhibits receptors for PDGF and VEGF, as well as other receptor tyrosine kinases such as CSF. While several phase II trials (NCT00514228, NCT00361309) have shown promising results[69,70], it is inferior to sorafenib and the latest phase III trial had to be terminated for safety reasons[71].
Orantinib is a receptor tyrosine kinase inhibitor that binds and inhibits the autophosphorylation of VEGFR2, PDGF-receptor and fibroblast growth factor receptor (FGFR), thereby inhibiting angiogenesis and cell proliferation. A phase I/II trial has shown a trend towards prolonged progression free survival in patients treated with orantinib after transarterial chemoembolization[72], and a phase III trial is still ongoing (NCT01465464). Blocking PDGF signaling in mouse models of pancreatic carcinogenesis with orantinib caused regression of blood vessels, as a result of the detachment of pericytes from tumor vessels. The fact that tumor vessels lacking pericytes are more vulnerable suggests that they could be more responsive to other anti-angiogenic drugs[73,74]. Combining receptor tyrosine kinase inhibitors targeting ECs and pericytes successfully diminished tumor angiogenesis and decreased tumor size compared to a monotherapeutic approach in colon cancer[75]. Similar effects were seen when PDGF inhibitors were combined with anti-angiogenic treatments[74]. Thus, targeting stellate cells and endothelial cells may destabilize the existing tumor vasculature more potently than targeting each cell type individually.
After liver damage, the pool of the liver’s resident macrophages-Kupffer cells-is rapidly expanded. A harmful incident causes the hepatic macrophages to secrete pro-inflammatory cytokines and chemokines such as IL-1β, TNF, CCL2, and CCL5, resulting in the activation of protective or apoptotic signaling pathways of hepatocytes and the recruitment of immune cells that support hepatic injury. There is increasing evidence suggesting that phagocytosis of apoptotic bodies by HSC and by macrophages may directly stimulate fibrogenesis through upregulation of TGF-β[76]. Furthermore, these repeated cycles of hepatocyte death and compensatory proliferation provide a mitogenic and mutagenic environment that fuels the development of HCC.
The location of Kupffer cells in the sinusoids allows close interactions with other non-parenchymal liver cells. Firstly, Kupffer cells interact with other immune cells by secreting inflammatory cytokines and chemokines. Secondly, they can activate HSC via paracrine mechanisms, likely involving TGF-β and PDGF. These profibrotic functions of Kupffer cells during chronic liver injury possibly contribute to a tumor-stimulating environment in the cirrhotic liver. In vivo studies have shown that depleting macrophages reduces angiogenesis and slows down tumor progression in mouse models, and enhances the response to sorafenib[77].
Macrophages can be classified into two main classes depending on their phenotypic polarization: the M1-phenotype, triggering a Th1 immune response and exerting cytotoxic activity; and the M2-phenotype, which activates a Th2 immune response and promotes angiogenesis, tissue remodeling and tumor progression[78]. Macrophages can adapt to signals from the microenvironment and change their functional phenotype accordingly[79]. M1 macrophages are activated as a response to microbial stimuli and interferon gamma, while in a tumor environment the tumour-associated macrophages (TAMs) are mainly polarized towards a M2 phenotype. Increased numbers of M2-macrophages have been associated with angiogenesis, metastasis and poor prognosis.
Tumor associated macrophages are key actors of cancer-related inflammation, being the main type of inflammatory cells infiltrating the tumor environment[80]. In HCC, tumor cells have been shown to recruit and activate TAMs by the secretion of VEGF, PlGF, PDGF, TGF-β and glypican-3. Glypican-3 is a member of the glypican family of heparin-sulfate proteoglycans linked to the cell surface. It is highly expressed in the majority of HCC cells and is known for its role in the regulation of cell proliferation and apoptosis[81]. In addition, studies have suggested an involvement in the recruitment of M2-polarized TAM’s in human HCC tissues[82]. Possibly glypican-3 present on the cell surface of malignant cells, binds to CCL5 and CCL3, which are chemokines that attract TAMs. Glypican-3 antibodies could therefore block the recruitment of TAMs via CCL5 and CCL3. Antibodies targeting glypican-3 have been tested in several phase I trials for advanced HCC, with promising results. The antibody was well tolerated and preliminary antitumor activity show a threefold prolongation of the median time to progression in patients receiving glypican-3-antibodies compared to untreated patients[83,84].
Zoledronic acid (ZA) is a compound widely used to prevent skeletal complications associated with bone metastases. Recent studies have shown a possible direct role as an anti-tumor agent by targeting the TAMs. ZA is taken up by macrophages via phagocytosis and leads to apoptosis specifically in TAMs, thus causing a repolarization of the macrophage population[85,86]. In vivo studies of ZA in combination with sorafenib have shown that the latter leads to an increase of M2-macrophages infiltrating the tumor stroma, which can be effectively depleted with ZA. This significantly inhibits angiogenesis, metastasis and tumor progression compared to sorafenib alone[77]. A phase II study of sorafenib and ZA in advanced HCC has been conducted (NCT01259193), but no results have been published.
Several studies have shown that the stroma regulates the malignant transformation, survival, progression and metastasis of hepatocellular carcinoma. Factors derived from the tumor cells in their turn alter the tumor stroma to generate a tumor-permissive microenvironment. This complex interplay between the tumor and the different actors in the stroma establishes a promising axis for therapeutic targets (Figure 2).
VEGF targeting therapies have represented the first success in treating HCC patients in many years, reviving research in this field and leading to an explosion of clinical trials with anti-angiogenic therapies[49]. However, the success of treatments such as sorafenib needs to be followed by better understanding of the mechanisms that underlie the intrinsic and acquired resistance to anti-angiogenic therapies. Perhaps targeting several actors of the stromal environment and the tumor cells at the same time could be the key for optimal treatment in future therapies.
Sorafenib does not only inhibit angiogenesis, but also alters the inflammatory environment. Sorafenib has been shown to suppress natural killer cells and facilitate tumor growth and metastasis[87]. Furthermore, multi-tyrosine kinase inhibitors have been shown to increase infiltration of tumor-associated macrophages in the tumor environment which could contribute to the resistance or escape to anti-angiogenic treatment[77] (although it is important to note that some studies have shown the opposite effect[88]). Hence the solution could be the use of adjuvant immunotherapy along with tyrosine kinase inhibitors for patients with unresectable HCC in order to obtain long-term response. In fact, one of the first trials to confirm the efficacy of sorafenib in advanced, metastasized renal cell carcinoma, was performed in combination with immunotherapy with IL-2 and interferon-alpha[89].
Tumor associated macrophages are important actors of cancer-related inflammation, being the main type of inflammatory cells infiltrating the tumor environment. Targeting macrophages as a therapeutic strategy could be done by depleting the overall population of macrophages, or by altering their phenotype from a M2 towards an M1 orientation. Again, macrophages are known to not only interact with the tumor cells and stimulate their growth, they also influence stellate cell activation and angiogenesis.
The stellate cells are one of the key players in the formation of the perfect tumor environment. Not only do they directly affect tumor growth by secreting growth factors[7], matrix proteases[8] and/or ECM proteins[9], they also alter the mechanical properties of the tumor’s surrounding. Activated stellate cells are known to stimulate angiogenesis, which allows the tumor cells to grow rapidly and invade in the circulatory system. The deposition of ECM proteins, such as collagens and proteoglycans, serve as a reservoir for growth factors, but also directly provoke a pro-tumoral cellular response. Indeed, the thick layer of ECM in the cirrhotic liver could impair drug delivery and hence decrease response to therapy. Therefore, preventing or reversing the activation of stellate cells could inhibit HCC growth, decrease angiogenesis and increase response to other therapies, such as classic chemotherapy or sorafenib.
As our understanding of the complex interplay between tumor and stroma evolves, the next-generation cancer drugs could target several actors in the tumor-stroma axis and offer a durable treatment for advanced HCC.
P- Reviewer: Meindl-Beinker NM, Tao R S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, Van Vlierberghe H. Angiogenesis in chronic liver disease and its complications. Liver Int. 2011;31:146-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 2. | Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013;123:3190-3200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 452] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 3. | Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473-1492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 501] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 4. | Otranto M, Sarrazy V, Bonté F, Hinz B, Gabbiani G, Desmoulière A. The role of the myofibroblast in tumor stroma remodeling. Cell Adh Migr. 2012;6:203-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Geng ZM, Li QH, Li WZ, Zheng JB, Shah V. Activated human hepatic stellate cells promote growth of human hepatocellular carcinoma in a subcutaneous xenograft nude mouse model. Cell Biochem Biophys. 2014;70:337-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Carloni V, Luong TV, Rombouts K. Hepatic stellate cells and extracellular matrix in hepatocellular carcinoma: more complicated than ever. Liver Int. 2014;34:834-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Amann T, Bataille F, Spruss T, Mühlbauer M, Gäbele E, Schölmerich J, Kiefer P, Bosserhoff AK, Hellerbrand C. Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci. 2009;100:646-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Coulouarn C, Corlu A, Glaise D, Guénon I, Thorgeirsson SS, Clément B. Hepatocyte-stellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma. Cancer Res. 2012;72:2533-2542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Santamato A, Fransvea E, Dituri F, Caligiuri A, Quaranta M, Niimi T, Pinzani M, Antonaci S, Giannelli G. Hepatic stellate cells stimulate HCC cell migration via laminin-5 production. Clin Sci (Lond). 2011;121:159-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Zhao W, Zhang L, Xu Y, Zhang Z, Ren G, Tang K, Kuang P, Zhao B, Yin Z, Wang X. Hepatic stellate cells promote tumor progression by enhancement of immunosuppressive cells in an orthotopic liver tumor mouse model. Lab Invest. 2014;94:182-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Zhao W, Zhang L, Yin Z, Su W, Ren G, Zhou C, You J, Fan J, Wang X. Activated hepatic stellate cells promote hepatocellular carcinoma development in immunocompetent mice. Int J Cancer. 2011;129:2651-2661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Campbell JS, Hughes SD, Gilbertson DG, Palmer TE, Holdren MS, Haran AC, Odell MM, Bauer RL, Ren HP, Haugen HS. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci USA. 2005;102:3389-3394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Campbell JS, Johnson MM, Bauer RL, Hudkins KL, Gilbertson DG, Riehle KJ, Yeh MM, Alpers CE, Fausto N. Targeting stromal cells for the treatment of platelet-derived growth factor C-induced hepatocellular carcinogenesis. Differentiation. 2007;75:843-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Nitta T, Kim JS, Mohuczy D, Behrns KE. Murine cirrhosis induces hepatocyte epithelial mesenchymal transition and alterations in survival signaling pathways. Hepatology. 2008;48:909-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Wright JH, Johnson MM, Shimizu-Albergine M, Bauer RL, Hayes BJ, Surapisitchat J, Hudkins KL, Riehle KJ, Johnson SC, Yeh MM. Paracrine activation of hepatic stellate cells in platelet-derived growth factor C transgenic mice: evidence for stromal induction of hepatocellular carcinoma. Int J Cancer. 2014;134:778-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Giannelli G, Villa E, Lahn M. Transforming growth factor-β as a therapeutic target in hepatocellular carcinoma. Cancer Res. 2014;74:1890-1894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 17. | Dooley S, Weng H, Mertens PR. Hypotheses on the role of transforming growth factor-beta in the onset and progression of hepatocellular carcinoma. Dig Dis. 2009;27:93-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Dooley S, Hamzavi J, Ciuclan L, Godoy P, Ilkavets I, Ehnert S, Ueberham E, Gebhardt R, Kanzler S, Geier A. Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology. 2008;135:642-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 19. | Bogaerts E, Heindryckx F, Vandewynckel YP, Van Grunsven LA, Van Vlierberghe H. The roles of transforming growth factor-β, Wnt, Notch and hypoxia on liver progenitor cells in primary liver tumours (Review). Int J Oncol. 2014;44:1015-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 843] [Cited by in F6Publishing: 854] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 21. | Dituri F, Mazzocca A, Peidro FJ, Papappicco P, Fabregat I, De Santis F, Paradiso A, Sabba C, Giannelli G. Differential Inhibition of the TGF-β Signaling Pathway in HCC Cells Using the Small Molecule Inhibitor LY2157299 and the D10 Monoclonal Antibody against TGF-β Receptor Type II. PLoS One. 2013;8:e67109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Gressner OA, Fang M, Li H, Lu LG, Gressner AM, Gao CF. Connective tissue growth factor (CTGF/CCN2) in serum is an indicator of fibrogenic progression and malignant transformation in patients with chronic hepatitis B infection. Clin Chim Acta. 2013;421:126-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Liu Y, Meyer C, Müller A, Herweck F, Li Q, Müllenbach R, Mertens PR, Dooley S, Weng HL. IL-13 induces connective tissue growth factor in rat hepatic stellate cells via TGF-β-independent Smad signaling. J Immunol. 2011;187:2814-2823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Li G, Xie Q, Shi Y, Li D, Zhang M, Jiang S, Zhou H, Lu H, Jin Y. Inhibition of connective tissue growth factor by siRNA prevents liver fibrosis in rats. J Gene Med. 2006;8:889-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Maass T, Thieringer FR, Mann A, Longerich T, Schirmacher P, Strand D, Hansen T, Galle PR, Teufel A, Kanzler S. Liver specific overexpression of platelet-derived growth factor-B accelerates liver cancer development in chemically induced liver carcinogenesis. Int J Cancer. 2011;128:1259-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Patel S. Long-term efficacy of imatinib for treatment of metastatic GIST. Cancer Chemother Pharmacol. 2013;72:277-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Lai JP, Sandhu DS, Shire AM, Roberts LR. The tumor suppressor function of human sulfatase 1 (SULF1) in carcinogenesis. J Gastrointest Cancer. 2008;39:149-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Lai JP, Thompson JR, Sandhu DS, Roberts LR. Heparin-degrading sulfatases in hepatocellular carcinoma: roles in pathogenesis and therapy targets. Future Oncol. 2008;4:803-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Parish CR, Freeman C, Brown KJ, Francis DJ, Cowden WB. Identification of sulfated oligosaccharide-based inhibitors of tumor growth and metastasis using novel in vitro assays for angiogenesis and heparanase activity. Cancer Res. 1999;59:3433-3441. [PubMed] [Cited in This Article: ] |
| 30. | Liu CJ, Lee PH, Lin DY, Wu CC, Jeng LB, Lin PW, Mok KT, Lee WC, Yeh HZ, Ho MC. Heparanase inhibitor PI-88 as adjuvant therapy for hepatocellular carcinoma after curative resection: a randomized phase II trial for safety and optimal dosage. J Hepatol. 2009;50:958-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Liu CJ, Chang J, Lee PH, Lin DY, Wu CC, Jeng LB, Lin YJ, Mok KT, Lee WC, Yeh HZ. Adjuvant heparanase inhibitor PI-88 therapy for hepatocellular carcinoma recurrence. World J Gastroenterol. 2014;20:11384-11393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 49] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Giannelli G, Fransvea E, Bergamini C, Marinosci F, Antonaci S. Laminin-5 chains are expressed differentially in metastatic and nonmetastatic hepatocellular carcinoma. Clin Cancer Res. 2003;9:3684-3691. [PubMed] [Cited in This Article: ] |
| 33. | Duan W, Ma J, Ma Q, Xu Q, Lei J, Han L, Li X, Wang Z, Wu Z, Lv S. The Activation of β1-integrin by Type I Collagen Coupling with the Hedgehog Pathway Promotes the Epithelial-Mesenchymal Transition in Pancreatic Cancer. Curr Cancer Drug Targets. 2014;. [PubMed] [Cited in This Article: ] |
| 34. | Sok JC, Lee JA, Dasari S, Joyce S, Contrucci SC, Egloff AM, Trevelline BK, Joshi R, Kumari N, Grandis JR. Collagen type XI α1 facilitates head and neck squamous cell cancer growth and invasion. Br J Cancer. 2013;109:3049-3056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Shields MA, Dangi-Garimella S, Redig AJ, Munshi HG. Biochemical role of the collagen-rich tumour microenvironment in pancreatic cancer progression. Biochem J. 2012;441:541-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 36. | Mailloux A, Epling-Burnette P. Collagen matrix deposition by hepatic stellate cells protects hepatocellular carcinoma from NK-mediated cytotoxicity. J Immunol. 2013;190. Available from:http: //www.jimmunol.org/cgi/content/meeting_abstract/190/1_MeetingAbstracts/53.7. [Cited in This Article: ] |
| 37. | Robert F, Gordon MS, Rosen LS, Mendelson DS, Mulay M, Adams BJ, Alvarez D, Theuer CP, Leigh BR. Final results from a phase I study of TRC093 (humanized anti-cleaved collagen antibody) in patients with solid cancer. J Clin Oncol. 2010;28:15. [Cited in This Article: ] |
| 38. | Gordon MS, Rosen LS, Robert F, Mendelson DS, Kleinzweig D, Adams BJ, Theuer CP. A phase 1, open-label, dose escalation study of the humanized monoclonal antibody (HuMAb) TRC093, an inhibitor of angiogenesis that binds to cleaved collagen, in patients with locally advanced or metastatic solid tumors. Ejc Supplements. 2008;6:130-130. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Wong GL. Transient elastography: Kill two birds with one stone? World J Hepatol. 2013;5:264-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Wang HM, Hung CH, Lu SN, Chen CH, Lee CM, Hu TH, Wang JH. Liver stiffness measurement as an alternative to fibrotic stage in risk assessment of hepatocellular carcinoma incidence for chronic hepatitis C patients. Liver Int. 2013;33:756-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Coulon S, Legry V, Heindryckx F, Van Steenkiste C, Casteleyn C, Olievier K, Libbrecht L, Carmeliet P, Jonckx B, Stassen JM. Role of vascular endothelial growth factor in the pathophysiology of nonalcoholic steatohepatitis in two rodent models. Hepatology. 2013;57:1793-1805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Heindryckx F, Coulon S, Terrie E, Casteleyn C, Stassen JM, Geerts A, Libbrecht L, Allemeersch J, Carmeliet P, Colle I. The placental growth factor as a target against hepatocellular carcinoma in a diethylnitrosamine-induced mouse model. J Hepatol. 2013;58:319-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Van Steenkiste C, Ribera J, Geerts A, Pauta M, Tugues S, Casteleyn C, Libbrecht L, Olievier K, Schroyen B, Reynaert H. Inhibition of placental growth factor activity reduces the severity of fibrosis, inflammation, and portal hypertension in cirrhotic mice. Hepatology. 2011;53:1629-1640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Hellström M, Gerhardt H, Kalén M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543-553. [PubMed] [Cited in This Article: ] |
| 45. | Zhan P, Qian Q, Yu LK. Serum VEGF level is associated with the outcome of patients with hepatocellular carcinoma: a meta-analysis. Hepatobiliary Surg Nutr. 2013;2:209-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 26] [Reference Citation Analysis (0)] |
| 46. | Tsuchiya K, Asahina Y, Matsuda S, Muraoka M, Nakata T, Suzuki Y, Tamaki N, Yasui Y, Suzuki S, Hosokawa T. Changes in plasma vascular endothelial growth factor at 8 weeks after sorafenib administration as predictors of survival for advanced hepatocellular carcinoma. Cancer. 2014;120:229-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | Huang GW, Yang LY, Lu WQ. Expression of hypoxia-inducible factor 1alpha and vascular endothelial growth factor in hepatocellular carcinoma: Impact on neovascularization and survival. World J Gastroenterol. 2005;11:1705-1708. [PubMed] [Cited in This Article: ] |
| 48. | Germano D, Daniele B. Systemic therapy of hepatocellular carcinoma: current status and future perspectives. World J Gastroenterol. 2014;20:3087-3099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Pircher A, Medinger M, Drevs J. Liver cancer: Targeted future options. World J Hepatol. 2011;3:38-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Boige V, Malka D, Bourredjem A, Dromain C, Baey C, Jacques N, Pignon JP, Vimond N, Bouvet-Forteau N, De Baere T. Efficacy, safety, and biomarkers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinoma. Oncologist. 2012;17:1063-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 51. | Hsu CH, Kang YK, Yang TS, Shun CT, Shao YY, Su WC, Sandoval-Tan J, Chiou TJ, Jin K, Hsu C. Bevacizumab with erlotinib as first-line therapy in Asian patients with advanced hepatocellular carcinoma: a multicenter phase II study. Oncology. 2013;85:44-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Britten CD, Gomes AS, Wainberg ZA, Elashoff D, Amado R, Xin Y, Busuttil RW, Slamon DJ, Finn RS. Transarterial chemoembolization plus or minus intravenous bevacizumab in the treatment of hepatocellular cancer: a pilot study. BMC Cancer. 2012;12:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Zhu AX, Finn RS, Mulcahy M, Gurtler J, Sun W, Schwartz JD, Dalal RP, Joshi A, Hozak RR, Xu Y. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin Cancer Res. 2013;19:6614-6623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 54. | Zhu AX, Ancukiewicz M, Supko JG, Sahani DV, Blaszkowsky LS, Meyerhardt JA, Abrams TA, McCleary NJ, Bhargava P, Muzikansky A. Efficacy, safety, pharmacokinetics, and biomarkers of cediranib monotherapy in advanced hepatocellular carcinoma: a phase II study. Clin Cancer Res. 2013;19:1557-1566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 55. | Alberts SR, Fitch TR, Kim GP, Morlan BW, Dakhil SR, Gross HM, Nair S. Cediranib (AZD2171) in patients with advanced hepatocellular carcinoma: a phase II North Central Cancer Treatment Group Clinical Trial. Am J Clin Oncol. 2012;35:329-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Lieu CH, Tran H, Jiang ZQ, Mao M, Overman MJ, Lin E, Eng C, Morris J, Ellis L, Heymach JV. The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. PLoS One. 2013;8:e77117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 57. | Miyahara K, Nouso K, Yamamoto K. Chemotherapy for advanced hepatocellular carcinoma in the sorafenib age. World J Gastroenterol. 2014;20:4151-4159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Tai WT, Cheng AL, Shiau CW, Liu CY, Ko CH, Lin MW, Chen PJ, Chen KF. Dovitinib induces apoptosis and overcomes sorafenib resistance in hepatocellular carcinoma through SHP-1-mediated inhibition of STAT3. Mol Cancer Ther. 2012;11:452-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 59. | Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, Hsu CH, Hu TH, Heo J, Xu J. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517-3524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 565] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 60. | Comito G, Calvani M, Giannoni E, Bianchini F, Calorini L, Torre E, Migliore C, Giordano S, Chiarugi P. HIF-1α stabilization by mitochondrial ROS promotes Met-dependent invasive growth and vasculogenic mimicry in melanoma cells. Free Radic Biol Med. 2011;51:893-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 61. | Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1813] [Cited by in F6Publishing: 1857] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 62. | Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 309] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 63. | Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541-1545. [PubMed] [Cited in This Article: ] |
| 64. | Thomas HE, Mercer CA, Carnevalli LS, Park J, Andersen JB, Conner EA, Tanaka K, Matsutani T, Iwanami A, Aronow BJ. mTOR inhibitors synergize on regression, reversal of gene expression, and autophagy in hepatocellular carcinoma. Sci Transl Med. 2012;4:139ra84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 65. | Finn RS, Poon RT, Yau T, Klümpen HJ, Chen LT, Kang YK, Kim TY, Gomez-Martin C, Rodriguez-Lope C, Kunz T. Phase I study investigating everolimus combined with sorafenib in patients with advanced hepatocellular carcinoma. J Hepatol. 2013;59:1271-1277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 66. | Zhu AX, Abrams TA, Miksad R, Blaszkowsky LS, Meyerhardt JA, Zheng H, Muzikansky A, Clark JW, Kwak EL, Schrag D. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117:5094-5102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 67. | Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, Poon RTP, Blanc JF, Vogel A, Chen CL. EVOLVE-1: Phase 3 study of everolimus for advanced HCC that progressed during or after sorafenib. J Clin Oncol. 2014;32:3. [Cited in This Article: ] |
| 68. | Toh HC, Chen PJ, Carr BI, Knox JJ, Gill S, Ansell P, McKeegan EM, Dowell B, Pedersen M, Qin Q. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer. 2013;119:380-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 69. | Sahani DV, Jiang T, Hayano K, Duda DG, Catalano OA, Ancukiewicz M, Jain RK, Zhu AX. Magnetic resonance imaging biomarkers in hepatocellular carcinoma: association with response and circulating biomarkers after sunitinib therapy. J Hematol Oncol. 2013;6:51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Koeberle D, Montemurro M, Samaras P, Majno P, Simcock M, Limacher A, Lerch S, Kovàcs K, Inauen R, Hess V. Continuous Sunitinib treatment in patients with advanced hepatocellular carcinoma: a Swiss Group for Clinical Cancer Research (SAKK) and Swiss Association for the Study of the Liver (SASL) multicenter phase II trial (SAKK 77/06). Oncologist. 2010;15:285-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067-4075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 523] [Cited by in F6Publishing: 564] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 72. | Inaba Y, Kanai F, Aramaki T, Yamamoto T, Tanaka T, Yamakado K, Kaneko S, Kudo M, Imanaka K, Kora S. A randomised phase II study of TSU-68 in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Eur J Cancer. 2013;49:2832-2840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 418] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 74. | Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23:939-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 412] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 75. | Shaheen RM, Tseng WW, Davis DW, Liu W, Reinmuth N, Vellagas R, Wieczorek AA, Ogura Y, McConkey DJ, Drazan KE. Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms. Cancer Res. 2001;61:1464-1468. [PubMed] [Cited in This Article: ] |
| 76. | Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655-663. [PubMed] [Cited in This Article: ] |
| 77. | Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, Kong LQ, Wang L, Wu WZ, Tang ZY. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420-3430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 78. | Roderfeld M, Rath T, Lammert F, Dierkes C, Graf J, Roeb E. Innovative immunohistochemistry identifies MMP-9 expressing macrophages at the invasive front of murine HCC. World J Hepatol. 2010;2:175-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342-349. [PubMed] [Cited in This Article: ] |
| 80. | Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F, Alesse E. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 283] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 81. | Yao M, Wang L, Dong Z, Qian Q, Shi Y, Yu D, Wang S, Zheng W, Yao D. Glypican-3 as an emerging molecular target for hepatocellular carcinoma gene therapy. Tumour Biol. 2014;35:5857-5868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Takai H, Ashihara M, Ishiguro T, Terashima H, Watanabe T, Kato A, Suzuki M. Involvement of glypican-3 in the recruitment of M2-polarized tumor-associated macrophages in hepatocellular carcinoma. Cancer Biol Ther. 2009;8:2329-2338. [PubMed] [Cited in This Article: ] |
| 83. | Ikeda M, Ohkawa S, Okusaka T, Mitsunaga S, Kobayashi S, Morizane C, Suzuki I, Yamamoto S, Furuse J. Japanese phase I study of GC33, a humanized antibody against glypican-3 for advanced hepatocellular carcinoma. Cancer Sci. 2014;105:455-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 84. | Zhu AX, Gold PJ, El-Khoueiry AB, Abrams TA, Morikawa H, Ohishi N, Ohtomo T, Philip PA. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2013;19:920-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 85. | Rogers TL, Wind N, Hughes R, Nutter F, Brown HK, Vasiliadou I, Ottewell PD, Holen I. Macrophages as potential targets for zoledronic acid outside the skeleton-evidence from in vitro and in vivo models. Cell Oncol (Dordr). 2013;36:505-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Coscia M, Quaglino E, Iezzi M, Curcio C, Pantaleoni F, Riganti C, Holen I, Mönkkönen H, Boccadoro M, Forni G. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med. 2010;14:2803-2815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 87. | Zhang QB, Sun HC, Zhang KZ, Jia QA, Bu Y, Wang M, Chai ZT, Zhang QB, Wang WQ, Kong LQ. Suppression of natural killer cells by sorafenib contributes to prometastatic effects in hepatocellular carcinoma. PLoS One. 2013;8:e55945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Sprinzl MF, Reisinger F, Puschnik A, Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A, Galle PR, Schuchmann M. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology. 2013;57:2358-2368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 89. | Escudier B, Lassau N, Angevin E, Soria JC, Chami L, Lamuraglia M, Zafarana E, Landreau V, Schwartz B, Brendel E. Phase I trial of sorafenib in combination with IFN alpha-2a in patients with unresectable and/or metastatic renal cell carcinoma or malignant melanoma. Clin Cancer Res. 2007;13:1801-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 90. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9016] [Cited by in F6Publishing: 9515] [Article Influence: 594.7] [Reference Citation Analysis (1)] |
| 91. | Kanai F, Yoshida H, Tateishi R, Sato S, Kawabe T, Obi S, Kondo Y, Taniguchi M, Tagawa K, Ikeda M. A phase I/II trial of the oral antiangiogenic agent TSU-68 in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2011;67:315-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 92. | Park JW, Finn RS, Kim JS, Karwal M, Li RK, Ismail F, Thomas M, Harris R, Baudelet C, Walters I. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2011;17:1973-1983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 93. | El-Khoueiry A, Posey JA, Castillo Ferrando JR, Krishnamurthi SS, Syed S, Kollia G, Walters I, Fischer BS, Masson E. The effects of liver impairment on the pharmacokinetics of brivanib, a dual inhibitor of fibroblast growth factor receptor and vascular endothelial growth factor receptor tyrosine kinases. Cancer Chemother Pharmacol. 2013;72:53-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Buijs M, Reyes DK, Pawlik TM, Blackford AL, Salem R, Messersmith WA, Weekes CD, Mulcahy M, Kamel IR, Geschwind JF. Phase 2 trial of concurrent bevacizumab and transhepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. Cancer. 2013;119:1042-1049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 95. | Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, Poon RT, Blanc JF, Vogel A, Chen CL. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 457] [Article Influence: 45.7] [Reference Citation Analysis (0)] |