Published online Dec 27, 2013. doi: 10.4254/wjh.v5.i12.654
Revised: October 31, 2013
Accepted: December 9, 2013
Published online: December 27, 2013
Processing time: 200 Days and 11.6 Hours
Tumors of the biliary tree are relatively rare; but their incidence is rising worldwide. There are several known risk factors for bile duct cancers, and these are seem to be associated with chronic inflammation of the biliary epithelium. Herein, 2 risk factors have been discussed, primary sclerosing cholangitis and reflux of pancreatic juice into the bile duct, as seen in such as an abnormal union of the pancreatic-biliary junction because magnetic resonance imaging (MRI) is used widely and effectively in the diagnosis of these diseases. When biliary disease is suspected, MRI can often help differentiate between benignity and malignancy, stage tumors, select surgical candidates and guide surgical planning. MRI has many advantages over other modalities. Therefore, MRI is a reliable noninvasive imaging tool for diagnosis and pre-surgical evaluation of bile duct tumors. Nowadays remarkable technical advances in magnetic resonance technology have expanded the clinical applications of MRI in case of biliary diseases. In this article, it is also discussed how recent developments in MRI contributes to the diagnosis of the bile duct cancer and the evaluation of patients with risk factors affecting bile duct cancer.
Core tip: Tumors of the biliary tree are relatively rare; but their incidence is rising worldwide. When biliary disease is suspected, magnetic resonance imaging (MRI) can often help differentiate between benignity and malignancy, stage tumors, select surgical candidates and guide surgical planning. Nowadays remarkable technical advances in magnetic resonance technology have expanded the clinical applications of MRI in case of biliary diseases. In this article, it is also discussed how recent developments in MRI contributes to the diagnosis of the bile duct cancer and the evaluation of patients with risk factors affecting bile duct cancer.
- Citation: Sugita R. Magnetic resonance evaluations of biliary malignancy and condition at high-risk for biliary malignancy: Current status. World J Hepatol 2013; 5(12): 654-665
- URL: https://www.wjgnet.com/1948-5182/full/v5/i12/654.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i12.654
Bile duct malignancies are relatively rare, estimated at 2% of all cancers with an incidence of 0.01%-0.04% in autopsy series[1]; however their incidence is rising worldwide[2,3]. The several known risk factors account for bile duct cancers, and these seem to be associated with chronic inflammation of the biliary epithelium[4-7]. The exact mechanism of tumor development is not completely understood and various possible pathways have been proposed, including chronic inflammatory process in the bile duct, mutation, and parasite-induced DNA damage[4,7-11]. When biliary disease is suspected, optimal imaging studies provide the required information for differentiating between benign and malignant tumors, tumor staging, selection of surgical candidate, and surgical planning of bile duct cancer. Various imaging modalities, invasive and noninvasive, are employed in diagnosis and staging of bile duct tumors[1,12]. The invasive methods include endoscopic retrograde cholangiopancreatography (ERCP), endoscopic ultrasonography (EUS), intraductal ultrasonography (IDUS), percutaneous transhepatic cholangiography (PTC), and optical coherence tomography. Noninvasive imaging methods include ultrasonography (US), multidetector computed tomography (MDCT), magnetic resonance imaging (MRI), and positron emission tomography-computed tomography (PET-CT). ERCP and PTC are not used as diagnostic tools alone owing to invasive nature. Nowadays ERCP is used for interventions such as biopsy, drainage and EUS/IDUS. US, EUS and IDUS are useful technique for screening biliary diseases particularly gallbladder disease; however their efficacy depends on operator skill and experience. MDCT are accurate and useful imaging techniques for the evaluation of biliary diseases. MDCT offers detailed information about the biliary tree and surrounding structures; however, it has some demerits such as ionized radiation and adverse reaction of intravenous contrast materials. MRI is a reliable noninvasive common imaging tool for the diagnosis and pre-surgical evaluation of bile duct tumors. MRI has many advantages over other modalities: (1) it is completely noninvasive, does not require exposure to ionizing radiation, and does not cause patient discomfort; (2) it does not require expert technicians with sophisticated technical skills. Therefore MRI has become an important diagnostic tool for bile duct diseases.
Moreover nowadays remarkable technical advances in magnetic resonance (MR) technology have increased the clinical applications of MRI for diagnosing biliary diseases[12-15]. In this article, it is discussed how developments in MRI have improved the evaluation of patients with risk factor affecting bile duct cancers and the diagnosis of bile duct cancers.
A pre-procedural fasting is recommended for gallbladder distension and gastric emptying. When fluid is present in the stomach and duodenum, visualization of the bile duct may be obscured by interposition of bowel loop. Therefore administration of oral contrast agent (iron oxide particles, blueberry juice or pineapple juice) is recommended.
Most institutes may perform MR examinations at 1.5 T with a torso coil. Although imaging at 3 T can improve the signal-to-noise ratio and spatial resolution, it may be hampered by dielectric effects, banding, and other pulse sequence-related effects[16-18]. The pulse sequences used for MRI of the bile duct are usually axial T1- and T2-weighted imaging, MR cholangiopancreatography (MRCP), and axial diffusion-weighted imaging (DWI). T1-weighted image may be used under an intravenous contrast material. Most gadolinium contrast agents produce an enhancement pattern similar to that observed with iodine-based CT contrast. The advent of the hepatocyte-specific contrast agents (Gd-EOB-DTPA, Gd-BOPTA, etc.) allows the usual early-phase imaging of the arterial, portal, and venous phases, plus delayed-phase hepatic parenchymal and biliary imaging, taking advantage of the fact that about 50% of injected dose of these contrast agents are excreted via the biliary system[19,20].
MRCP use 2 varieties of T2-weighted sequences. One is obtained with a single-shot turbo spin-echo T2-weighted sequence by using a long echo time to selectively display the fluid filled bile ducts. The other is obtained by using a navigator-based respiratory-triggered three-dimensional acquisition sequence with a longer acquisition time[21]. The differences of both are small, and thus either or both are used for MRCP accordingly.
DWI can obtain additional information derived from the microscopic motion of proton in water, which is not possible by using conventional MRI. DWI is a sensitive sequence for the detection of tumors and inflammation of the bile ducts. It has the advantage of quantitative data analysis through the generation of apparent diffusion coefficient (ADC) maps, which can contribute to objective disease assessment and monitoring of response to therapy[22-25].
MRI can allow us to evaluate the analysis of bile and pancreatic juice flow, which may have relate to carcinogenesis of the bile duct tumors. Although by now the flow analysis of the bile duct based on MRI was held by a continuous MRCP examination after secretin injection, a new method [time-spatial labeling inversion pulse (SLIP) imaging] become to evaluate the flow analysis easier and faster than before[26].
Risk factors for bile duct carcinoma include (1) primary sclerosing cholangitis (PSC), (2) reflux of pancreatic juice into the common bile duct, such as in an abnormal arrangement of the pancreato-biliary ductal system (AAPB), (3) exposure to chemicals, and (4) medication such as oral contraceptives and methyldopa[4-7]. In this chapter, MRI applications for benign biliary diseases and condition at a high-risk for malignancy are discussed about PSC and reflux of pancreatic juice into the bile duct because MRI is used widely and effectively for these entities (Table 1).
| MR characteristics | Differential diagnosis | Comparison to other modalities | Sensitivity and specificity | Pitfall of MRI | |
| PSC | Diffuse stricture and/or beaded appearance of the bile duct on MRCP | Cholangitis, Cholangiocarcinoma | ERCP is considered the standard method. MRCP is considered being sufficient for diagnosis of PSC | High sensitivity and very high specificity | It is often difficult to differentiate malignant tumors from PSC |
| Cholan- giocarci- noma | MRI with MRCP is usually considered the modality of choice in the diagnosis of cholangiocarcinoma | Diagnosis of biliary stenosis by MRCP is high sensitivity and specificity. The ability of differentiation between benign obstruction and malignant is low | Minimal invasion along the mucosa and in the perineural space is difficult to diagnose | ||
| Intrahepatic cholangio- carcinoma | The tumor shows an irregular shaped solid mass with peripheral rim enhancement and incomplete concentric pooling of contrast material on dynamic study | Metastasis, Mixed HCC, cholangiocellular carcinoma | |||
| Extrahepatic cholangio- carcinoma | The most common pattern of the tumor growth is focal infiltration of the ductal wall or the periductal-infiltrating type, resulting in focal strictures | PSC, cholangitis (IgG4, infection, AIDS), sarcoidosis | |||
| Gallbladder carcinoma | In the diffusely infiltrative type, the tumor appears as a large solid mass in the gallbladder fossa In the polypoid and mural thickening types, lesion more than 10 mm in diameter or which enhance after intravenous contrast material, are usually malignant | Polyp, adenomyomatosis, xanthogranulomatous cholecystitis, chronic cholecystitis | Usually, US is used as an initial diagnostic modality As a second step, CT, MRI with MRCP, and /or traditional cholangiography is often used for obtaining additional information | Conventional MRI showed 74% of sensitivity and 68%-83% specificity, while DWI set added to conventional MRI showed high sensitivity and specificity | It is often difficult malignant from benign tumors |
| Ampullary carcinoma | It is difficult to diagnose because of the small tumor on MRI. DWI has the potential for differentiating malignant from benign ampullary tumors | Cholangiocarcinoma, Pancreas cancer, adenoma, inflammatory diseases, carcinoid | MRI with MRCP is more accurate than CT in differentiating between malignant and benign lesions | High sensitivity (100%) and low specificity (59.1%-63.6%). Adding of DWI to conventional MRI improve specificity | It is often difficult to diagnose because of the small tumor |
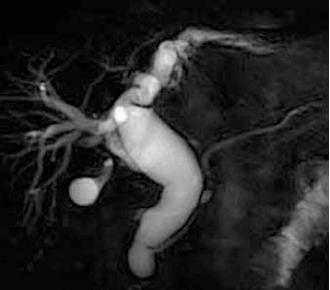
PSC: PSC is a chronic cholestatic liver disease of possible autoimmune origin, characterized by intra- and extrahepatic bile duct inflammation and fibrosis[4,27-31]. PSC is the most common risk factor for cholangiocarcinoma in Western countries, with a prevalence of cholangiocarcinoma ranging from 8% to 25%[27]. Diagnostic criteria for PSC include (1) typical cholangiographic abnormalities; (2) clinical, biochemical, and hepatic histologic finding; and (3) the exclusion of secondary cause of sclerosing cholangitis.
The diagnosis of PSC was based on characteristic cholagiographic finding in combination with clinical, biochemical, and histologic features. Therefore ERCP was considered the standard method for diagnosis of PSC. However, owing to developments in MR technology, MRCP has become another important modality[32-41]. The result of a meta-analysis showed that MRCP had high sensitivity and very high specificity for the diagnosis of PSC[33] (Figure 1). The radiological characteristics of PSC mimic those of cholangiocarcinoma[42]. Both make differential diagnosis quite difficult even with current diagnostic modalities including MRI.
AAPB: AAPB is a congenital anomaly defined as the junction of the pancreatic and bile ducts being located outside the duodenal wall. As the contraction of the sphincter of Oddi within the duodenal wall does not functionally affect the junction in patients with this congenital abnormality, continuous pancreaticobiliary reflux occurs, resulting in a high incidence of biliary cancer. AAPB can be divided into (1) AAPB with biliary dilatation (choledochal cyst) and (2) AAPB without biliary dilatation.
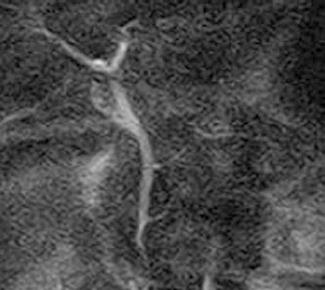
AAPB with choledochal cyst: Choledochal cysts are rare congenital biliary tract anomalies characterized by biliary tree dilatation. Although the incidence in the Western population is 1 in 100000 to 150000 live births, it is much higher in Asian countries, particularly Japan, where they can be found in up to 1 in 1000 live birth[43-45]. Choledochal cysts are usually classified into several types, based on anatomical findings. According to Todani’s classification system, choledochal cysts include five main types.
In Todani’s classification system, almost all patients with choledochal cyst are classified into 3 types (type Ia, Ic and IV-A), and that associated with AAPB. Biliary tract malignancies were seen in 10%-30% of patients with choledochal cyst and it increases with age[45]. A prompt and accurate diagnosis of choledochal cyst, follow by surgical is therefore essential.
In diagnostic imaging, researchers have shown that MRCP can offer diagnostic information equivalent to that of ERCP for assessment of choledochal cysts in adults[46,47] (Figure 2). Although MRCP should not replace ERCP totally in pediatric patients, MRCP should be considered the first-choice imaging technique for evaluation of choledochal cysts. MRCP can provide preoperative information about minute structure of AAPB in children with choledochal cysts[48].
AAPB without choledochal cyst: AAPB patients without choledochal cyst, similar to those with choledochal cyst, experience continuous reciprocal reflux between pancreatic juice and bile[49]. Because the hydro pressure within the pancreatic duct is usually greater than that within the bile duct, pancreatic juice frequently refluxes into the bile duct in these patients, which results in a high incidence of cancer of the biliary tract.
Although AAPB patients with and without choledochal cyst have a risk of biliary malignancy, the usual sites of malignancy differ. To the contrast bile duct and gallbladder cancers were seen in 34% and 65% of AAPB with choledochal cysts, only gallbladder cancer was found in almost all of 38% of AAPB without biliary dilatation[50]. Once AAPB is diagnosed, prophylactic flow-diversion surgery (bile duct resection and biloenteric anastomosis) is performed for patients with choledochal cyst.
Treatment of patients with AAPB without biliary dilatation is controversial. Prophylactic cholecystectomy is performed in many institutions. However, some surgeons propose excision of the extrahepatic bile duct, together with gallbladder.
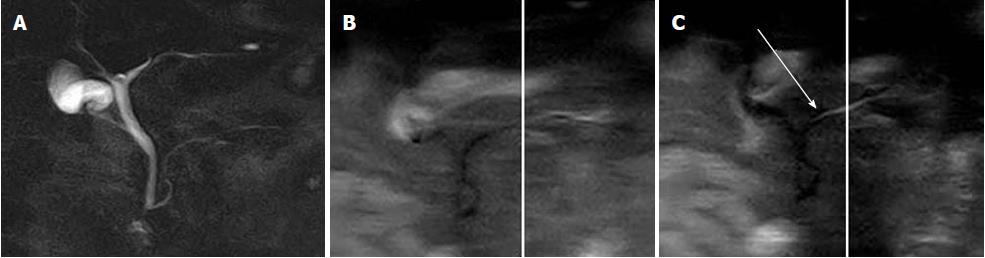
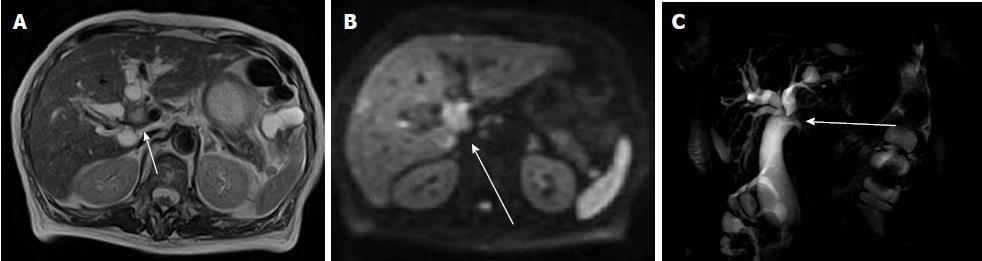
The diagnostic criteria for AAPB have been established on the basis of ERCP. Although Kamisawa et al[50] have shown that MRCP can be used to detect AAPB (Figure 3), they have reported that some atypical cases with relative short common channel cannot be diagnosed by MRCP, and should be confirmed by ERCP.
AAPB cases with choledochal cysts have clinical symptoms due to cholangitis or pancreatitis in childhood, and thus they tend to be diagnosed in childhood. Patients without choledochal cysts are usually not diagnosed until adulthood, when they have already progressed to advanced stage gallbladder carcinoma, which has a poor prognosis. An appropriate strategy is necessary to detect and manage these cases. Takuma et al[51] have suggested that MRCP should be performed in patients who are found to have gallbladder wall thickening by US.
Recently, several case series have been published on the reflux of pancreatic juice into the bile duct without a morphologically AAPB, and the correlation of such cases with biliary diseases, especially biliary malignancies, is drawing attention[52-57]. These cases could not detected by existing imaging modalities based on morphological change.
Several reports have shown that high amylase levels in bile samples on ERCP, which indicate reflux of pancreatic juice, or reflux of contrast medium into the pancreatic duct during intraoperative cholangiography, were found in 26%-87% of patients with normal pancreaticobiliary duct anatomy[58,59].
Several reports have revealed that MRCP can be used to detect pancreatic juice reflux in those patients[53,55]. In patients without AAPB, reflux of pancreatic juice into the common bile duct can be indirectly observed by using secretin-stimulating MRCP. The cause of such reflux may be dysfunction of the sphincter of Oddi.
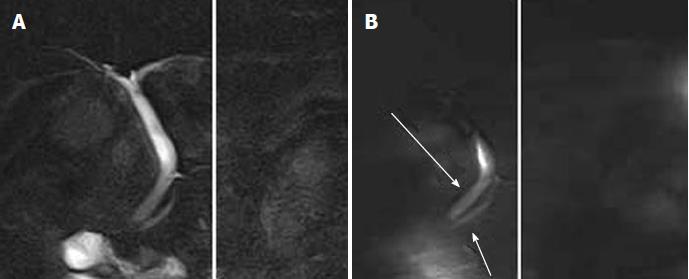
The new method of time-SLIP technique, used in vascular studies, has the potential to visualize pancreatic juice flow directly[26] (Figure 4). Researchers have shown that this method can be used to detect pancreatic juice flow reflux in the normal patients (Figure 5). The new technique may reveal more information on the rate of pancreaticobiliary reflux in the population with normal biliary anatomy and help determine whether is associated with an increased incidence of biliary malignancy.
In general, the diagnosis of biliary tumors, particularly early detection and differential diagnosis, is still challenging, although many sensitive direct and indirect techniques have been adopted.
Cholangiocarcinoma arise from the epithelial cells lining the biliary tree. Intrahepatic cholangiocarcinoma arise within the intrahepatic ducts and extrahepatic cholangiocarcinoma originate in the bile duct along the hepato-duodenal ligament. Extrahepatic biliary carcinomas are further divided into hilar, also called Klatskin tumors, and distal tumors. Hilar tumors represent approximately 60%-70% of cholangiocarcinoma, distal tumors represent 20%-30%, and intrahepatic cholangiocarcinomas represent 5%-10%[1,4,5].
The tumors are rare, estimated at 3% of all gastrointestinal cancers. They are the second most common type of primary hepatic tumors[4,7,8]. This ratio includes intrahepatic and extrahepatic tumors. The patients present mostly in the 6th and 7th decades of life.
The pathologic classification of cholangiocarcinoma categorize into 3 types: mass-forming, periductal infiltrating, and intraductal growing[60]. The intraductal growing type is currently thought to be the counterpart of intraductal papillary mucinous neoplasm of the pancreas[13,61-67].
MRI with MRCP is usually considered the modality of choice for the diagnosis of cholangiocarcinomas. Several studies have shown that MRI has sensitivity and specificity > 90%. However, its ability to differentiate between benign and malignant obstruction is low and variable, according to the authors[68].
Intrahepatic cholangiocarcinoma: Intrahepatic cholangiocarcinoma is the second most common primary hepatic malignant tumors after hepatocellular carcinoma[13,68,69]. The important prognostic factors of intrahepatic cholangiocarcinoma are tumor size, lymph node metastasis, and vascular invasion.
The mass-forming type makes up a large percentage of intrahepatic cholangiocarcinoma, and shows an irregular shaped solid mass with peripheral rim enhancement and incomplete concentric pooling of contrast material on dynamic studies[13,70-72]. The MRI appearances depend on the degree of fibrosis, coagulative necrosis, cell debris, and mucin production. Capsular retraction, bile duct dilatation distal to the tumor, vascular encasement, and central scar have been also reported.
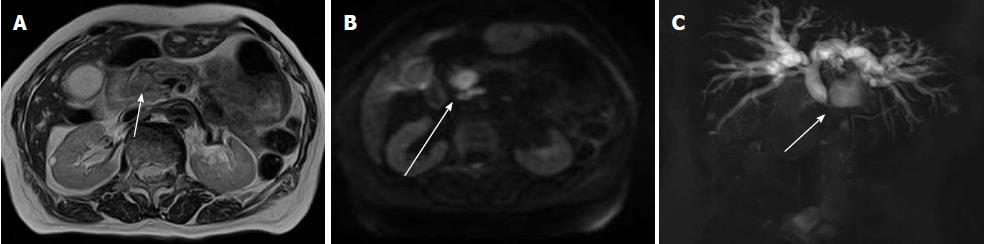
Several researchers have reported that the use of hepatocyte-specific contrast agent (Gd-EOB-DTPA) may aid in the diagnosis of intrahepatic cholangiocarcinoma[73-76]. They have shown that Gd-EOB-DTPA enhanced images displayed increased lesion conspicuity and better delineation of daughter nodules and intrahepatic metastases. Other researchers have reported that DWI may be also useful for detection of bile duct cancers[77,78] (Figure 6).
Extrahepatic biliary cancer: Extrahepatic biliary carcinomas are divided into hilar, also called Klatskin tumors, and distal tumors. Hilar tumors represent approximately 60%-70% and distal tumors 20%-30%[4,5]. The most common pattern of tumor growth is focal infiltration of the ductal wall or the periductal-infiltrating type, resulting in focal strictures. The mass-forming and intraductal-growing types are less common[13].
The role of MRI is to detect and characterize the tumor, and determine respectability. On cross-sectional MRI, the lesion appears ill-defined, and moderately hypo- to isointense on T1-weighted images and mildly iso- to hyperintense on T2-weighted images as compared to adjacent liver parenchyma.
Hilar bile duct cancers are most commonly of the infiltrative type and less frequently exophytic or polypoid lesions[13,14]. Many studies have reported that MRI, including MRCP, is useful in the staging of perihilar bile duct cancers[79-84] (Figure 7). MRI cannot assess tumor in stented ducts[81,82]. Minimal invasion along the mucosa and in the perineural space may escape detection if it is below the limit of resolution[82,83].
Distal extrahepatic cholangiocarcinomas are most commonly of the infiltrative type and grow intramurally, beneath the bile duct epithelium. The accuracy of MRCP is reported to be comparable to that of ERCP for differentiating extrahepatic bile duct carcinoma from benign stricture[60,85-92]. Although some overlap exists, in general the presence of a long segment of extrahepatic bile duct stricture with irregular margins and asymmetric narrowing is suggestive of cholangiocarcinoma, whereas a short segment with regular margins and symmetric narrowing indicates a benign cause[87]. The addition of a contrast-enhanced dynamic study to evaluate the longitudinal tumor extent of bile duct cancers is controversial. One report has shown favorable results, but another report showed no improvement in diagnostic accuracy[93,94].
Several researchers have reported on the utility of DWI in these lesions, and it may play an important role in the diagnosis of extrahepatic tumors[95,96] (Figure 8).
Primary carcinoma of the gallbladder is the most common malignancy of the biliary tract. Spread of gallbladder carcinoma to the liver is common due to the thinness of the gallbladder’s smooth muscular layer and the proximity to the liver, allowing spread to lymphatic channels[97-101]. Gallbladder carcinomas exhibit 3 typical patterns: polypoid, mural thickening, and diffusely infiltrative[102]. Nearly 70% of gallbladder carcinoma present as diffusely infiltrative lesions[97].
Usually, US is used as an initial diagnostic modality. As a second step, CT, MRI with MRCP, and/or traditional cholangiography is often used for obtaining additional information. Comparative studies of CT and MRI with MRCP are desirable.
The role of MRI is to characterize the tumor, and determine respectability[103,104]. Gallbladder carcinoma usually exhibits low to intermediate signal intensity on T1-weighted sequences and heterogenous hyperintensity on T2-weighted sequences with a characteristically ill-defined contour[105]. In the polypoid and mural thickening types, lesion more than 10 mm in diameter or which enhance after intravenous contrast material, are usually malignant. The diffusely infiltrative type, the tumor appears as a large solid mass in the gallbladder fossa, obscuring the gallbladder. The presence of gallstones within the mass may be helpful in making the diagnosis. In tumor staging, differentiation between stage T1 (lesions confined to the muscular layer) and stage T2 (lesions confined to subserosal or perimuscular connective tissue) is important, because vastly different operative procedures used depending on the stage. Yoshimitsu et al[101] have reported that submucosal enhancement on a delayed phase dynamic MRI study is a useful sign for differentiating between the stages.
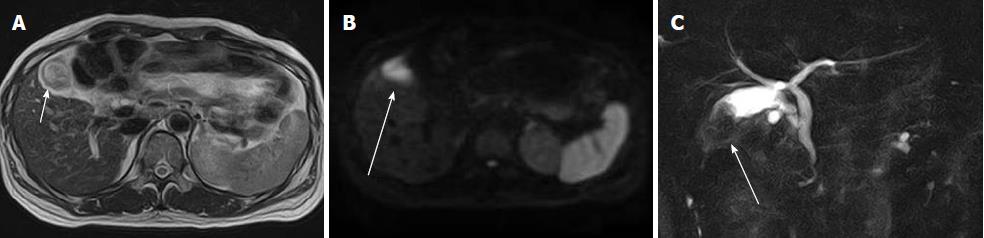
Several researchers have showed that DWI may be useful in the diagnosis of gallbladder carcinoma[106-109] (Figure 9). The sensitivity and specificity of conventional MRI alone was 74% and 68%-83%, respectively; these values increased when DWI was used along with conventional MRI[24].
Ampullary carcinoma tends to appear as small mass that causes biliary obstruction. Although CT and MRI are used to evaluate ampullary carcinoma, it is difficult to diagnose because of the small tumors and difficulty of differentiating between the tumors and surrounding normal structure. MRI, including MRCP, has been reported to be more accurate than CT[110,111]. MRI in ampullary carcinoma has a high sensitivity and low specificity[112]. EUS and ERCP are usually used to identify ampullary carcinoma.
Histologically, most ampullary carcinoma develop from 1 of 2 types of epithelium, resulting in an intestinal-type adenocarcinoma arising from the intestinal epithelium lining the duodenal papilla and pancreaticobiliary-type adenocarcinoma developing from the biliary epithelium of the ampullary portion. The subtypes of ampullary tumors have different prognoses. Chung et al[113] have shown MRI may be helpful in determining the subtypes of ampullary tumors.
Several studies have reported that DWI has the potential for differentiating malignant ampullary tumors from benign ampullary tumors[114,115]. Researchers have reported that malignant tumors have a low ADC value compared to that of benign tumors (Figure 10).
MRI is a promising non-invasive imaging technique for evaluating biliary lesions. MRI can be used for diagnosis, tumor characterization, preoperative planning, and follow-up of malignant biliary lesions.
P- Reviewers: Midorikawa Y, Thuwajit P, Wongkham S S- Editor: Zhai HH L- Editor: A E- Editor: Wu HL
| 1. | Ganeshan D, Moron FE, Szklaruk J. Extrahepatic biliary cancer: New staging classification. World J Radiol. 2012;4:345-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 846] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 3. | Watanapa P. Cholangiocarcinoma in patients with opisthorchiasis. Br J Surg. 1996;83:1062-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3:33-42. [PubMed] |
| 6. | Parkin DM, Ohshima H, Srivatanakul P, Vatanasapt V. Cholangiocarcinoma: epidemiology, mechanisms of carcinogenesis and prevention. Cancer Epidemiol Biomarkers Prev. 1993;2:537-544. [PubMed] |
| 7. | Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994;14:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 209] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Menias CO, Surabhi VR, Prasad SR, Wang HL, Narra VR, Chintapalli KN. Mimics of cholangiocarcinoma: spectrum of disease. Radiographics. 2008;28:1115-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Noda Y, Fujita N, Kobayashi G, Ito K, Horaguchi J, Takasawa O, Obana T, Ishida K, Senoo S, Yonechi M. Histological study of gallbladder and bile duct epithelia in patients with anomalous arrangement of the pancreaticobiliary ductal system: comparison between those with and without a dilated common bile duct. J Gastroenterol. 2007;42:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Zen Y, Quaglia A, Heaton N, Rela M, Portmann B. Two distinct pathways of carcinogenesis in primary sclerosing cholangitis. Histopathology. 2011;59:1100-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990-2009. World J Gastroenterol. 2009;15:4240-4262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 186] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 13. | Chung YE, Kim MJ, Park YN, Choi JY, Pyo JY, Kim YC, Cho HJ, Kim KA, Choi SY. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics. 2009;29:683-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 14. | Matos C, Serrao E, Bali MA. Magnetic resonance imaging of biliary tumors. Magn Reson Imaging Clin N Am. 2010;18:477-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Manfredi R, Barbaro B, Masselli G, Vecchioli A, Marano P. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Isoda H, Kataoka M, Maetani Y, Kido A, Umeoka S, Tamai K, Koyama T, Nakamoto Y, Miki Y, Saga T. MRCP imaging at 3.0 T vs. 1.5 T: preliminary experience in healthy volunteers. J Magn Reson Imaging. 2007;25:1000-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Li N, Liu C, Bi W, Lin X, Jiao H, Zhao P. MRCP and 3D LAVA imaging of extrahepatic cholangiocarcinoma at 3 T MRI. Clin Radiol. 2012;67:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Chang KJ, Kamel IR, Macura KJ, Bluemke DA. 3.0-T MR imaging of the abdomen: comparison with 1.5 T. Radiographics. 2008;28:1983-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Lee NK, Kim S, Lee JW, Lee SH, Kang DH, Kim GH, Seo HI. Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics. 2009;29:1707-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Koelblinger C, Schima W, Weber M, Mang T, Nemec S, Kulinna-Cosentini C, Bastati N, Ba-Ssalamah A. Gadoxate-enhanced T 1-weighted MR cholangiography: comparison of 1.5 T and 3.0 T. Rofo. 2009;181:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Choi JY, Kim MJ, Lee JM, Lee JY, Kim SH, Kim KW, Han JK, Choi BI. Magnetic resonance cholangiography: comparison of two- and three-dimensional sequences for assessment of malignant biliary obstruction. Eur Radiol. 2008;18:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Miquel ME, Scott AD, Macdougall ND, Boubertakh R, Bharwani N, Rockall AG. In vitro and in vivo repeatability of abdominal diffusion-weighted MRI. Br J Radiol. 2012;85:1507-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Lee NK, Kim S, Kim GH, Kim DU, Seo HI, Kim TU, Kang DH, Jang HJ. Diffusion-weighted imaging of biliopancreatic disorders: correlation with conventional magnetic resonance imaging. World J Gastroenterol. 2012;18:4102-4117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Kim SJ, Lee JM, Kim H, Yoon JH, Han JK, Choi BI. Role of diffusion-weighted magnetic resonance imaging in the diagnosis of gallbladder cancer. J Magn Reson Imaging. 2013;38:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Corona-Villalobos CP, Pan L, Halappa VG, Bonekamp S, Lorenz CH, Eng J, Kamel IR. Agreement and reproducibility of apparent diffusion coefficient measurements of dual-b-value and multi-b-value diffusion-weighted magnetic resonance imaging at 1.5 Tesla in phantom and in soft tissues of the abdomen. J Comput Assist Tomogr. 2013;37:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Sugita R, Furuta A, Horaguchi J, Itoh K, Kobayashi G, Noda Y, Fujita N, Shimizu S, Miyazaki M, Takahashi S. Visualization of pancreatic juice movement using unenhanced MR imaging with spin labeling: preliminary results in normal and pathophysiologic conditions. J Magn Reson Imaging. 2012;35:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Aljiffry M, Renfrew PD, Walsh MJ, Laryea M, Molinari M. Analytical review of diagnosis and treatment strategies for dominant bile duct strictures in patients with primary sclerosing cholangitis. HPB (Oxford). 2011;13:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Mendes FD, Lindor KD. Primary sclerosing cholangitis. Clin Liver Dis. 2004;8:195-211. [DOI] [Full Text] |
| 29. | Rosen CB, Nagorney DM, Wiesner RH, Coffey RJ, LaRusso NF. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg. 1991;213:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 231] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Lutz HH, Tischendorf JJ. Management of primary sclerosing cholangitis. World J Hepatol. 2011;3:137-141. [PubMed] |
| 31. | Lichtenstein DR. Hepatobiliary complications of inflammatory bowel disease. Curr Gastroenterol Rep. 2011;13:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Vitellas KM, Keogan MT, Freed KS, Enns RA, Spritzer CE, Baillie JM, Nelson RC. Radiologic manifestations of sclerosing cholangitis with emphasis on MR cholangiopancreatography. Radiographics. 2000;20:959-975; quiz 1108-1109, 1112. [PubMed] |
| 33. | Dave M, Elmunzer BJ, Dwamena BA, Higgins PD. Primary sclerosing cholangitis: meta-analysis of diagnostic performance of MR cholangiopancreatography. Radiology. 2010;256:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 34. | Vitellas KM, El-Dieb A, Vaswani KK, Bennett WF, Tzalonikou M, Mabee C, Kirkpatrick R, Bova JG. MR cholangiopancreatography in patients with primary sclerosing cholangitis: interobserver variability and comparison with endoscopic retrograde cholangiopancreatography. AJR Am J Roentgenol. 2002;179:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Weber C, Kuhlencordt R, Grotelueschen R, Wedegaertner U, Ang TL, Adam G, Soehendra N, Seitz U. Magnetic resonance cholangiopancreatography in the diagnosis of primary sclerosing cholangitis. Endoscopy. 2008;40:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Vitellas KM, Enns RA, Keogan MT, Freed KS, Spritzer CE, Baillie J, Nelson RC. Comparison of MR cholangiopancreatographic techniques with contrast-enhanced cholangiography in the evaluation of sclerosing cholangitis. AJR Am J Roentgenol. 2002;178:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Berstad AE, Aabakken L, Smith HJ, Aasen S, Boberg KM, Schrumpf E. Diagnostic accuracy of magnetic resonance and endoscopic retrograde cholangiography in primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2006;4:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Fulcher AS, Turner MA, Franklin KJ, Shiffman ML, Sterling RK, Luketic VA, Sanyal AJ. Primary sclerosing cholangitis: evaluation with MR cholangiography-a case-control study. Radiology. 2000;215:71-80. [PubMed] |
| 39. | Ferrara C, Valeri G, Salvolini L, Giovagnoni A. Magnetic resonance cholangiopancreatography in primary sclerosing cholangitis in children. Pediatr Radiol. 2002;32:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Textor HJ, Flacke S, Pauleit D, Keller E, Neubrand M, Terjung B, Gieseke J, Scheurlen C, Sauerbruch T, Schild HH. Three-dimensional magnetic resonance cholangiopancreatography with respiratory triggering in the diagnosis of primary sclerosing cholangitis: comparison with endoscopic retrograde cholangiography. Endoscopy. 2002;34:984-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Meagher S, Yusoff I, Kennedy W, Martel M, Adam V, Barkun A. The roles of magnetic resonance and endoscopic retrograde cholangiopancreatography (MRCP and ERCP) in the diagnosis of patients with suspected sclerosing cholangitis: a cost-effectiveness analysis. Endoscopy. 2007;39:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Clayton RA, Clarke DL, Currie EJ, Madhavan KK, Parks RW, Garden OJ. Incidence of benign pathology in patients undergoing hepatic resection for suspected malignancy. Surgeon. 2003;1:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Mortelé KJ, Rocha TC, Streeter JL, Taylor AJ. Multimodality imaging of pancreatic and biliary congenital anomalies. Radiographics. 2006;26:715-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 44. | Toki A, Suzuki J, Watarai Y, Sugiyama A, Hotta H, Nakayama T, Tanaka A. Is the classification of congenital biliary dilatation and pancreatobiliary maljunction useful (Japanese language)? Tan to Sui. 2012;33:17-22 Available from: http://www.igakutosho.co.jp/magazine/t_s. |
| 45. | Jabłońska B. Biliary cysts: etiology, diagnosis and management. World J Gastroenterol. 2012;18:4801-4810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 46. | Matos C, Nicaise N, Devière J, Cassart M, Metens T, Struyven J, Cremer M. Choledochal cysts: comparison of findings at MR cholangiopancreatography and endoscopic retrograde cholangiopancreatography in eight patients. Radiology. 1998;209:443-448. [PubMed] |
| 47. | Irie H, Honda H, Jimi M, Yokohata K, Chijiiwa K, Kuroiwa T, Hanada K, Yoshimitsu K, Tajima T, Matsuo S. Value of MR cholangiopancreatography in evaluating choledochal cysts. AJR Am J Roentgenol. 1998;171:1381-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Kim MJ, Han SJ, Yoon CS, Kim JH, Oh JT, Chung KS, Yoo HS. Using MR cholangiopancreatography to reveal anomalous pancreaticobiliary ductal union in infants and children with choledochal cysts. AJR Am J Roentgenol. 2002;179:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Kamisawa T, Takuma K, Itokawa F, Itoi T. Endoscopic diagnosis of pancreaticobiliary maljunction. World J Gastrointest Endosc. 2011;3:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kamata N. MRCP of congenital pancreaticobiliary malformation. Abdom Imaging. 2007;32:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Takuma K, Kamisawa T, Tabata T, Hara S, Kuruma S, Inaba Y, Kurata M, Honda G, Tsuruta K, Horiguchi S. Importance of early diagnosis of pancreaticobiliary maljunction without biliary dilatation. World J Gastroenterol. 2012;18:3409-3414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Horaguchi J, Fujita N, Noda Y, Kobayashi G, Ito K, Takasawa O, Obana T, Endo T, Nakahara K, Ishida K. Amylase levels in bile in patients with a morphologically normal pancreaticobiliary ductal arrangement. J Gastroenterol. 2008;43:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Motosugi U, Ichikawa T, Araki T, Kitahara F, Sato T, Itakura J, Fujii H. Secretin-stimulating MRCP in patients with pancreatobiliary maljunction and occult pancreatobiliary reflux: direct demonstration of pancreatobiliary reflux. Eur Radiol. 2007;17:2262-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Sai JK, Suyama M, Kubokawa Y, Tadokoro H, Sato N, Maehara T, Iida Y, Kojima K. Occult pancreatobiliary reflux in patients with a normal pancreaticobiliary junction. Gastrointest Endosc. 2003;57:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Sai JK, Ariyama J, Suyama M, Kubokawa Y, Sato N. Occult regurgitation of pancreatic juice into the biliary tract: diagnosis with secretin injection magnetic resonance cholangiopancreatography. Gastrointest Endosc. 2002;56:929-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Anderson MC, Hauman RL, Suriyapa C, Schiller WR. Pancreatic enzyme levels in bile of patients with extrahepatic biliary tract disease. Am J Surg. 1979;137:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Itokawa F, Itoi T, Nakamura K, Sofuni A, Kakimi K, Moriyasu F, Tsuchida A, Aoki T. Assessment of occult pancreatobiliary reflux in patients with pancreaticobiliary disease by ERCP. J Gastroenterol. 2004;39:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Beltrán MA. Current knowledge on pancreaticobiliary reflux in normal pancreaticobiliary junction. Int J Surg. 2012;10:190-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Kamisawa T, Anjiki H, Egawa N, Kurata M, Honda G, Tsuruta K. Diagnosis and clinical implications of pancreatobiliary reflux. World J Gastroenterol. 2008;14:6622-6626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 60. | Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR Am J Roentgenol. 2003;181:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 215] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 61. | Lim JH, Zen Y, Jang KT, Kim YK, Nakanuma Y. Cyst-forming intraductal papillary neoplasm of the bile ducts: description of imaging and pathologic aspects. AJR Am J Roentgenol. 2011;197:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Kim H, Lim JH, Jang KT, Kim MJ, Lee J, Lee JY, Choi D, Lim HK, Choi DW, Lee JK. Morphology of intraductal papillary neoplasm of the bile ducts: radiologic-pathologic correlation. Abdom Imaging. 2011;36:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Oki H, Hayashida Y, Namimoto T, Aoki T, Korogi Y, Yamashita Y. Usefulness of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance cholangiography for detecting mucin retention in bile ducts: a rare intraductal papillary mucinous neoplasm of the bile duct. Jpn J Radiol. 2011;29:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Park MS, Yu JS, Lee DK, Yoon DS, Cha SW, Kim KW. Gadobenate dimeglumine-enhanced MRI of intraductal papillary mucinous tumor of the bile ducts. J Magn Reson Imaging. 2007;25:625-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 282] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 66. | Zen Y, Fujii T, Itatsu K, Nakamura K, Konishi F, Masuda S, Mitsui T, Asada Y, Miura S, Miyayama S. Biliary cystic tumors with bile duct communication: a cystic variant of intraductal papillary neoplasm of the bile duct. Mod Pathol. 2006;19:1243-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 67. | Lim JH, Yoon KH, Kim SH, Kim HY, Lim HK, Song SY, Nam KJ. Intraductal papillary mucinous tumor of the bile ducts. Radiographics. 2004;24:53-66; discussion 66-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 68. | Van Beers BE. Diagnosis of cholangiocarcinoma. HPB (Oxford). 2008;10:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Slattery JM, Sahani DV. What is the current state-of-the-art imaging for detection and staging of cholangiocarcinoma? Oncologist. 2006;11:913-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 70. | Maetani Y, Itoh K, Watanabe C, Shibata T, Ametani F, Yamabe H, Konishi J. MR imaging of intrahepatic cholangiocarcinoma with pathologic correlation. AJR Am J Roentgenol. 2001;176:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 71. | Yoshida Y, Imai Y, Murakami T, Nishikawa M, Kurokawa M, Yonezawa T, Tokunaga K, Fukushima Y, Wakasa K, Kim T. Intrahepatic cholangiocarcinoma with marked hypervascularity. Abdom Imaging. 1999;24:66-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Rimola J, Forner A, Reig M, Vilana R, de Lope CR, Ayuso C, Bruix J. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology. 2009;50:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 73. | Kim SH, Lee CH, Kim BH, Kim WB, Yeom SK, Kim KA, Park CM. Typical and atypical imaging findings of intrahepatic cholangiocarcinoma using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging. J Comput Assist Tomogr. 2012;36:704-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Péporté AR, Sommer WH, Nikolaou K, Reiser MF, Zech CJ. Imaging features of intrahepatic cholangiocarcinoma in Gd-EOB-DTPA-enhanced MRI. Eur J Radiol. 2013;82:e101-e106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Kang Y, Lee JM, Kim SH, Han JK, Choi BI. Intrahepatic mass-forming cholangiocarcinoma: enhancement patterns on gadoxetic acid-enhanced MR images. Radiology. 2012;264:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 76. | Hwang J, Kim YK, Park MJ, Lee MH, Kim SH, Lee WJ, Rhim HC. Differentiating combined hepatocellular and cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma using gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2012;36:881-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Halappa VG, Bonekamp S, Corona-Villalobos CP, Li Z, Mensa M, Reyes D, Eng J, Bhagat N, Pawlik TM, Geschwind JF. Intrahepatic cholangiocarcinoma treated with local-regional therapy: quantitative volumetric apparent diffusion coefficient maps for assessment of tumor response. Radiology. 2012;264:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Yang DM, Jahng GH, Kim HC, Jin W, Ryu CW, Nam DH, Lee YK, Park SY. The detection and discrimination of malignant and benign focal hepatic lesions: T2 weighted vs diffusion-weighted MRI. Br J Radiol. 2011;84:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 79. | Vogl TJ, Schwarz WO, Heller M, Herzog C, Zangos S, Hintze RE, Neuhaus P, Hammerstingl RM. Staging of Klatskin tumours (hilar cholangiocarcinomas): comparison of MR cholangiography, MR imaging, and endoscopic retrograde cholangiography. Eur Radiol. 2006;16:2317-2325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Masselli G, Manfredi R, Vecchioli A, Gualdi G. MR imaging and MR cholangiopancreatography in the preoperative evaluation of hilar cholangiocarcinoma: correlation with surgical and pathologic findings. Eur Radiol. 2008;18:2213-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 81. | Choi JY, Kim MJ, Lee JM, Kim KW, Lee JY, Han JK, Choi BI. Hilar cholangiocarcinoma: role of preoperative imaging with sonography, MDCT, MRI, and direct cholangiography. AJR Am J Roentgenol. 2008;191:1448-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 82. | Masselli G, Gualdi G. Hilar cholangiocarcinoma: MRI/MRCP in staging and treatment planning. Abdom Imaging. 2008;33:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 83. | Chryssou E, Guthrie JA, Ward J, Robinson PJ. Hilar cholangiocarcinoma: MR correlation with surgical and histological findings. Clin Radiol. 2010;65:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Ruys AT, van Beem BE, Engelbrecht MR, Bipat S, Stoker J, Van Gulik TM. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol. 2012;85:1255-1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 85. | Romagnuolo J, Bardou M, Rahme E, Joseph L, Reinhold C, Barkun AN. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;139:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 263] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 86. | Kim MJ, Mitchell DG, Ito K, Outwater EK. Biliary dilatation: differentiation of benign from malignant causes--value of adding conventional MR imaging to MR cholangiopancreatography. Radiology. 2000;214:173-181. [PubMed] |
| 87. | Park MS, Kim TK, Kim KW, Park SW, Lee JK, Kim JS, Lee JH, Kim KA, Kim AY, Kim PN. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: findings at MRCP versus ERCP. Radiology. 2004;233:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 170] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 88. | Nandalur KR, Hussain HK, Weadock WJ, Wamsteker EJ, Johnson TD, Khan AS, D’Amico AR, Ford MK, Nandalur SR, Chenevert TL. Possible biliary disease: diagnostic performance of high-spatial-resolution isotropic 3D T2-weighted MRCP. Radiology. 2008;249:883-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 89. | Lee DH, Lee JM, Kim KW, Park HS, Kim SH, Lee JY, Han JK, Choi BI. MR imaging findings of early bile duct cancer. J Magn Reson Imaging. 2008;28:1466-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | Chung YE, Kim MJ, Park YN, Lee YH, Choi JY. Staging of extrahepatic cholangiocarcinoma. Eur Radiol. 2008;18:2182-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Park HS, Lee JM, Choi JY, Lee MW, Kim HJ, Han JK, Choi BI. Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. AJR Am J Roentgenol. 2008;190:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 92. | Kim JE, Lee JM, Kim SH, Baek JH, Moon SK, Yu IS, Kim SH, Lee JY, Han JK, Choi BI. Differentiation of intraductal growing-type cholangiocarcinomas from nodular-type cholangiocarcinomas at biliary MR imaging with MR cholangiography. Radiology. 2010;257:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Kim HJ, Lee JM, Kim SH, Han JK, Lee JY, Choi JY, Kim KH, Kim JY, Lee MW, Kim SJ. Evaluation of the longitudinal tumor extent of bile duct cancer: value of adding gadolinium-enhanced dynamic imaging to unenhanced images and magnetic resonance cholangiography. J Comput Assist Tomogr. 2007;31:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 94. | Ryoo I, Lee JM, Chung YE, Park HS, Kim SH, Han JK, Choi BI. Gadobutrol-enhanced, three-dimensional, dynamic MR imaging with MR cholangiography for the preoperative evaluation of bile duct cancer. Invest Radiol. 2010;45:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 95. | Cui XY, Chen HW. Role of diffusion-weighted magnetic resonance imaging in the diagnosis of extrahepatic cholangiocarcinoma. World J Gastroenterol. 2010;16:3196-3201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Cui XY, Chen HW, Cai S, Bao J, Tang QF, Wu LY, Fang XM. Diffusion-weighted MR imaging for detection of extrahepatic cholangiocarcinoma. Eur J Radiol. 2012;81:2961-2965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Sons HU, Borchard F, Joel BS. Carcinoma of the gallbladder: autopsy findings in 287 cases and review of the literature. J Surg Oncol. 1985;28:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 67] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 98. | Kelly TR, Chamberlain TR. Carcinoma of the gallbladder. Am J Surg. 1982;143:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 99. | Sumiyoshi K, Nagai E, Chijiiwa K, Nakayama F. Pathology of carcinoma of the gallbladder. World J Surg. 1991;15:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 100. | Levy AD, Murakata LA, Rohrmann CA. Gallbladder carcinoma: radiologic-pathologic correlation. Radiographics. 2001;21:295-314; questionnaire, 549-455. [PubMed] |
| 101. | Yoshimitsu K, Nishihara Y, Okamoto D, Ushijima Y, Nishie A, Yamaguchi K, Taketomi A, Honda H. Magnetic resonance differentiation between T2 and T1 gallbladder carcinoma: significance of subserosal enhancement on the delayed phase dynamic study. Magn Reson Imaging. 2012;30:854-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 102. | Yoshimitsu K, Honda H, Kaneko K, Kuroiwa T, Irie H, Ueki T, Chijiiwa K, Takenaka K, Masuda K. Dynamic MRI of the gallbladder lesions: differentiation of benign from malignant. J Magn Reson Imaging. 1997;7:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 103. | Kim JH, Kim TK, Eun HW, Kim BS, Lee MG, Kim PN, Ha HK. Preoperative evaluation of gallbladder carcinoma: efficacy of combined use of MR imaging, MR cholangiography, and contrast-enhanced dual-phase three-dimensional MR angiography. J Magn Reson Imaging. 2002;16:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 104. | Schwartz LH, Black J, Fong Y, Jarnagin W, Blumgart L, Gruen D, Winston C, Panicek DM. Gallbladder carcinoma: findings at MR imaging with MR cholangiopancreatography. J Comput Assist Tomogr. 2002;26:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | Catalano OA, Sahani DV, Kalva SP, Cushing MS, Hahn PF, Brown JJ, Edelman RR. MR imaging of the gallbladder: a pictorial essay. Radiographics. 2008;28:135-55; quiz 324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 106. | Sugita R, Yamazaki T, Furuta A, Itoh K, Fujita N, Takahashi S. High b-value diffusion-weighted MRI for detecting gallbladder carcinoma: preliminary study and results. Eur Radiol. 2009;19:1794-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 107. | Irie H, Kamochi N, Nojiri J, Egashira Y, Sasaguri K, Kudo S. High b-value diffusion-weighted MRI in differentiation between benign and malignant polypoid gallbladder lesions. Acta Radiol. 2011;52:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 108. | Ogawa T, Horaguchi J, Fujita N, Noda Y, Kobayashi G, Ito K, Koshita S, Kanno Y, Masu K, Sugita R. High b-value diffusion-weighted magnetic resonance imaging for gallbladder lesions: differentiation between benignity and malignancy. J Gastroenterol. 2012;47:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 109. | Sugita R, Ito K, Fujita N, Takahashi S. Diffusion-weighted MRI in abdominal oncology: clinical applications. World J Gastroenterol. 2010;16:832-836. [PubMed] |
| 110. | Andersson M, Kostic S, Johansson M, Lundell L, Asztély M, Hellström M. MRI combined with MR cholangiopancreatography versus helical CT in the evaluation of patients with suspected periampullary tumors: a prospective comparative study. Acta Radiol. 2005;46:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 111. | Wu DS, Chen WX, Wang XD, Acharya R, Jiang XH. Pancreaticobiliary duct changes of periampullary carcinomas: quantitative analysis at MR imaging. Eur J Radiol. 2012;81:2112-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 112. | Chung YE, Kim MJ, Kim HM, Park MS, Choi JY, Hong HS, Kim KW. Differentiation of benign and malignant ampullary obstructions on MR imaging. Eur J Radiol. 2011;80:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 113. | Chung YE, Kim MJ, Park MS, Choi JY, Kim H, Kim SK, Lee M, Kim HJ, Choi JS, Song SY. Differential features of pancreatobiliary- and intestinal-type ampullary carcinomas at MR imaging. Radiology. 2010;257:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 114. | Jang KM, Kim SH, Lee SJ, Park HJ, Choi D, Hwang J. Added value of diffusion-weighted MR imaging in the diagnosis of ampullary carcinoma. Radiology. 2013;266:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 115. | Lee NK, Kim S, Seo HI, Kim DU, Woo HY, Kim TU. Diffusion-weighted MR imaging for the differentiation of malignant from benign strictures in the periampullary region. Eur Radiol. 2013;23:1288-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |