INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is a highly malignant and progressive hepatobiliary carcinoma accounting for about 5%-15% of primary malignant liver tumors[1-3]. A majority of ICCs are classified as well to moderately differentiated adenocarcinoma, and once ICC shows invasive growth, it aggressively invades the surrounding tissue and is commonly associated with distant metastasis. Because it is often diagnosed at an advanced stage, ICC has a poor prognosis[2-4]. While ICC usually arises in an apparently normal liver, chronic cholangitis such as hepatolithiasis and primary sclerosing cholangitis, occasionally precedes its development[3-5]. While the incidence of ICC is gradually increasing in the Eastern and Western world, the mechanism underlying the malignant progression is not well understood.
Recently, studies on hepatolithiasis have identified two distinct neoplastic biliary intraepithelial lesions preceding invasive ICC[3]. The first is a flat or micropapillary growth of atypical biliary epithelium recognizable under a microscope, which has been called “biliary dysplasia”[5], and corresponds to “biliary intraepithelial neoplasia (BilIN)”, one of the precursor lesions of ICC in the World Health Organization’s classification of tumors[6]. The other is grossly characterized by the prominent papillary growth of atypical biliary epithelium with distinct fibrovascular cores and not-infrequent mucin over-production, which was recently termed an intraductal papillary neoplasm of the bile duct (IPN-B)[7,8]. BilIN and IPNB are now regarded as biliary counterparts of pancreatic intraepithelial neoplasia and pancreatic IPMN[9,10].
There is increasing evidence that the development and progression of malignant tumors of the biliary tree and their precursor lesions are associated with multiple alterations of cell-regulatory genes such as oncogenes and anti-oncogenes[11]. Phenotypic changes such as mucin and cytokeratin expression have also been reported in ICC and its precursor lesions[12,13]. In this review, we will discuss the recent progress in studies on the precursor lesions of ICC with an emphasis on histopathologic changes and phenotypic expression, as well as molecular and genetic changes. For a better understanding of these issues, the anatomy of the biliary tree and clinicopathological classification of ICC will be briefly reviewed.
ANATOMY OF THE BILIARY TREE AND CLASSIFICATION OF ICC
Anatomy of the biliary tree
The biliary tree is divided into the intrahepatic, hilar (right and left hepatic ducts and their confluence), and extrahepatic bile ducts. The intrahepatic bile duct is a branch of the right or left hepatic duct, and the first to third branches of both hepatic ducts are known as the intrahepatic large bile ducts which are grossly visible. Intrahepatic small bile ducts are the branches of intrahepatic large bile ducts, and are classified into septal and interlobular bile ducts. Bile ductules are located at the periphery of the portal tracts[14].
Clinicopathological classification of ICC
ICC is usually divided into either the peripheral type or the perihilar type, according to the location of the tumor along the biliary tree[1,2,15,16]. Perihilar type ICC is presumed to arise from the intrahepatic large bile ducts, though the ones arising from both hepatic bile ducts and their confluence could also be included in this group when they are invasive. Peripheral ICC arises in the hepatic parenchyma. The growth pattern of ICC is also grossly classified as being mass-forming, infiltrating periductally, or growing intraductally[6,17]. A majority of ICCs are well to moderately differentiated adenocarcinomas, and, in addition, colloid carcinoma is also occasionally identifiable. A majority of perihilar ICCs show periductal infiltration or nodular type along the biliary tree, and to a lesser degree, intraductal growth, while almost all peripheral ICCs are of a mass-forming type.
HISTOPATHOLOGICAL FEATURES OF PRECURSOR LESIONS OF INVASIVE ICC ARISING IN LARGE BILE DUCTS: FLAT AND PAPILLARY LESIONS
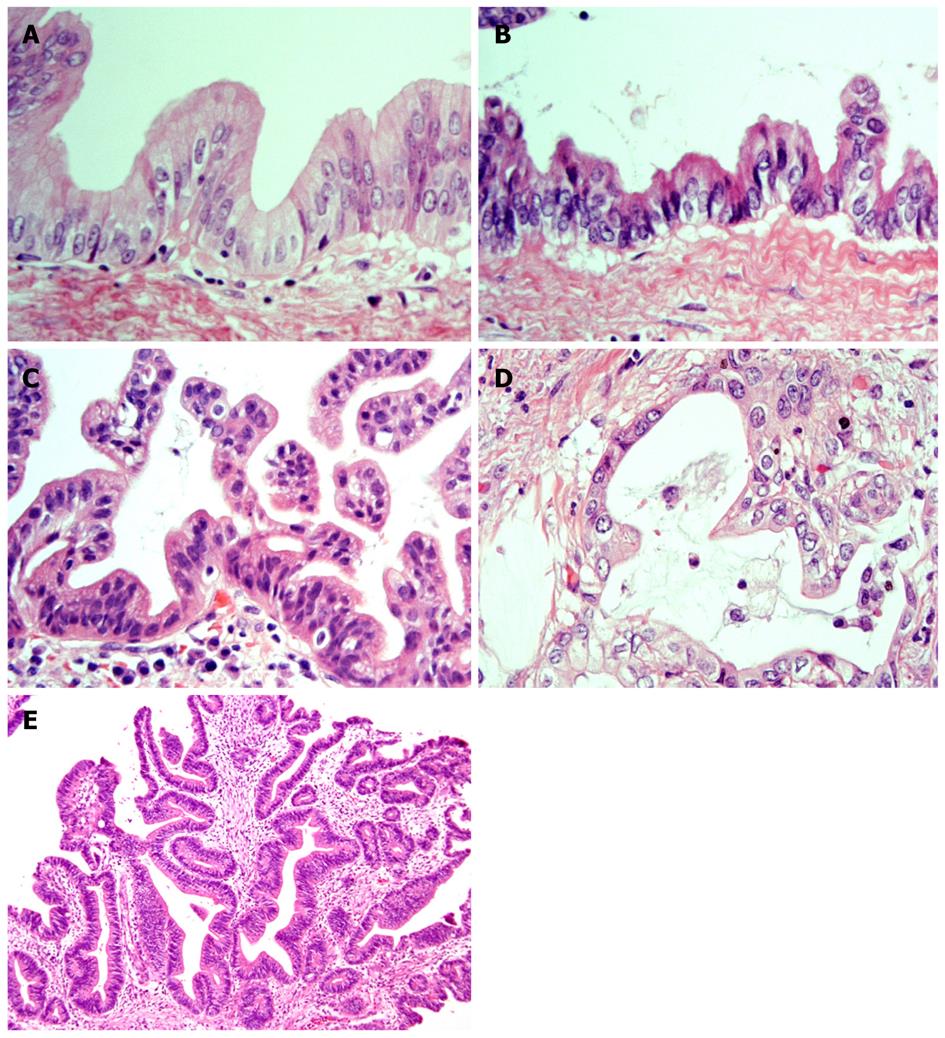
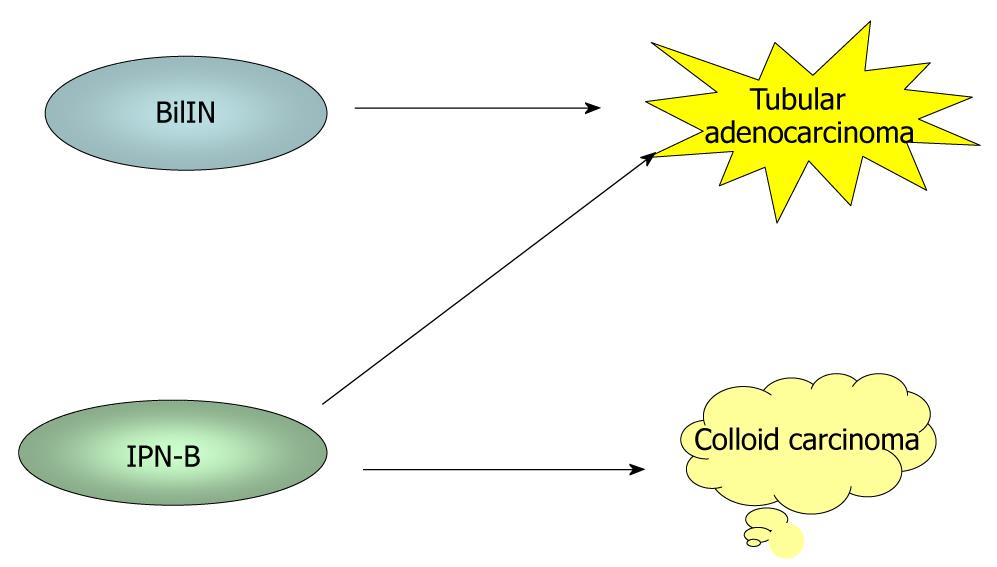
It has recently become evident that ICCs undergo a multistep-carcinogenesis, and two types of precursor lesions of invasive ICCs have been proposed: a flat intraepithelial neoplasia (BilIN) (Figure 1A-D) and an intraductal papillary lesion (IPNB) (Figure 1E)[6,7,9,18,19]. While the former is identifiable under a microscope, the latter is identifiable on radiologic images or through macroscopic examination[6,7]. These two lesions usually occur in the large intrahepatic bile ducts, hilar bile ducts and extrahepatic bile ducts, are rarely present in the septal or interlobular bile ducts. In addition, BilIN and IPN-B occur more frequently during chronic inflammatory biliary diseases, such as hepatolithiasis, primary sclerosing cholangitis, infestation by liver flukes, as well as congenital biliary diseases[6-8,20]. When accompanied by invasive lesions, BilIN is known to progress to conventional ICC (tubular adenocarcinoma), whereas IPN-B is associated with colloid carcinoma (mucinous carcinoma) or conventional ICC (Figure 2)[7,8,21].
Figure 1 Representative histological features of neoplasms (HE staining).
A: BilIN-1; B: BilIN-2; BilIN-3; D: Invasive ICC; E: IPN-B. BilIN: biliary intraepithelial neoplasm; ICC: intrahepatic cholangiocarcinoma; IPN-B: intraductal papillary neoplasm of the bile duct[22].
Figure 2 During biliary multistep carcinogenesis, BilIN is proposed to progresses to conventional ICC (tubular adenocarcinoma), whereas IPN-B is associated with colloid carcinoma (mucinous carcinoma) or conventional ICC.
While the exact carcinogenetic processes via these two precursor lesions remains to be clarified, recent studies have shown that, during their carcinogenetic pathways, different phenotypic or genetic changes take place[14,18,22-25].
Characteristics of BilIN lineages
BilIN is histologically defined as a flat or micropapillary proliferation of atypical biliary epithelium, showing multilayering, piled-up nuclei, an increased nucleocytoplasmic ratio, a partial loss of nuclear polarity, and nuclear hyperchromasia and pleomorphism. BilINs are now classified three grades: BilIN-1 (corresponding to low-grade dysplasia), BilIN-2 (high-grade dysplasia), and BilIN-3 (carcinoma in situ) (Figure 1A-D)[9,19]. Briefly, BilIN-1 shows mild cellular/nuclear atypia such as nuclear membrane irregularity or nuclear enlargement with only a minimal disturbance of cellular polarity. BilIN-2 has evident cellular/nuclear atypia, but not enough to suggest an overt carcinoma, with a focal disturbance of cellular polarity. BilIN-3 shows a diffuse disturbance of cellular polarity with or without distinct cellular/nuclear atypia corresponding to an overt carcinoma, though there is no invasion beyond the basement membrane. In this review, BilIN-2 and BilIN-3 are grouped together as high-grade BilIN (BilIN-2/3). ICCs with BilIN are histologically tubular adenocarcinomas with micropapillary components, when present.
Characteristics of IPNB lineages
IPN-B has been defined as a prominent papillary proliferation of atypical biliary epithelium with distinct fibrovascular cores, showing nuclear stratification, piled-up nuclei, and nuclear enlargement (Figure 1E)[7,8,18]. Some parts show tubular components as well. IPN-B shows grossly visible papillary lesions in the saccular or cystically dilated bile ducts. All colloid carcinomas with IPN-B characteristically show macronodular lesions in contrast to the nodular growth or periductal infiltrative spread of tubular adenocarcinoma with BilIN or IPN-B. Because there are no well-established criteria for grading IPN-B, we graded IPN-B according to the World Health Organization’s criteria for intraductal papillary mucinous neoplasm of the pancreas (IPMN-P)[26]. IPN-Bs were classified into two subgroups: IPN-B1 (corresponding to benign and borderline lesions of IPMN-P) and IPN-B2 (corresponding to carcinoma in situ). Interestingly, invasive IPN-B is of either the colloid type with variable amounts of tubular adenocarcinoma, or tubular adenocarcinoma without colloid components. Oncocytic papillary adenocarcinoma is also found in invasive areas. Recently, Shibahara et al[27] examined mucin-producing bile duct tumors, which are closely related to the IPN-B in this study, and reported that patients with tubular adenocarcinoma had a significantly lower rate of survival than those with mucinous (colloid) carcinoma.
EXPRESSION OF MUCIN CORE PROTEINS (MUCS) AND CYTOKERATINS (CKS) IN BILIN AND IPNB
Normal and pathologic intrahepatic bile ducts show a rather characteristic mucin and CK profile[10]. Physiologically, MUC3 and CK7 are expressed in the lining epithelium of biliary epithelial cells, though their expression is altered under pathologic conditions[14,28,29]. Interestingly, intestinal metaplasia (goblet cell metaplasia or Paneth cell metaplasia) is more frequent in cases of IPN-B than BilIN. In contrast, gastric metaplasia (foveolar or pseudopyloric gland metaplasia) was similarly observed in he BilIN and IPN-B lineages.
Expression of MUCs
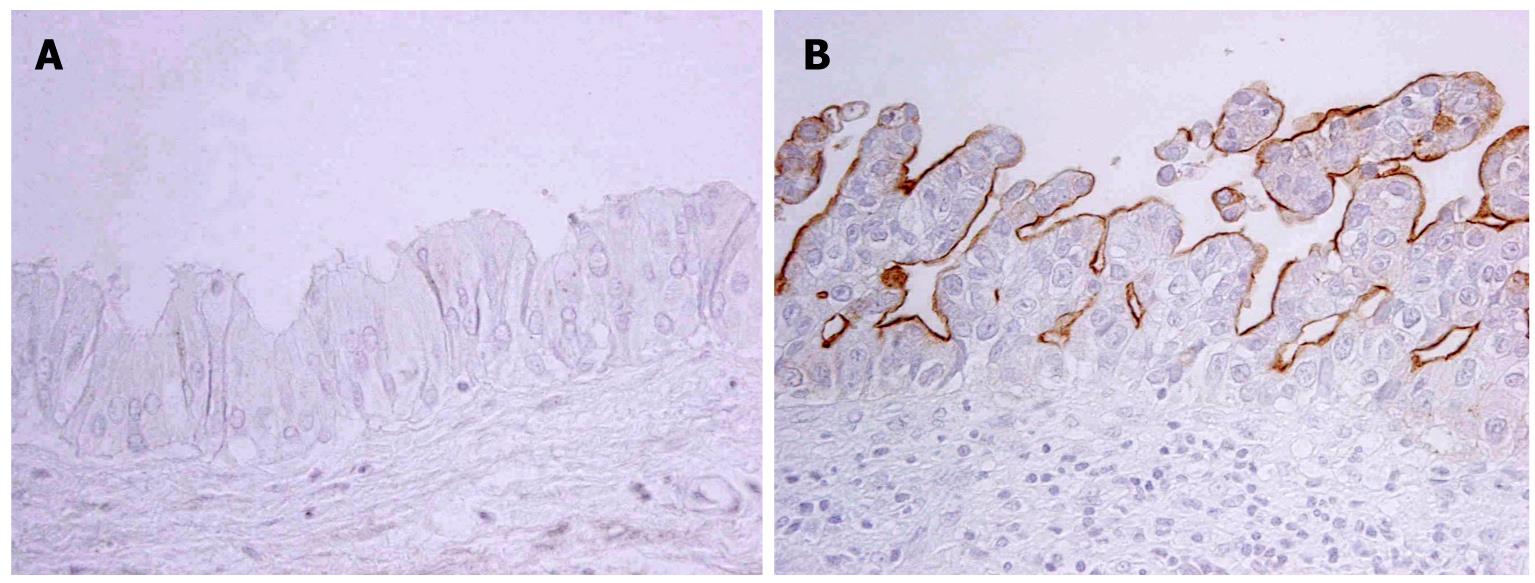
The expression profile of MUCs in neoplastic biliary epithelia differed according to the pathways of the BilIN or IPN-B lineage[10]. MUC1, usually not detectable in non-neoplastic biliary epithelial cells, is expressed in one fourth of BilINs, especially in high-grade lesions (Figure 3), while it is only occasionally expressed in IPN-Bs. MUC1 is expressed in more than half of tumor cells in all ICC cases with BilIN, but is expressed in less than half of ICC cases with IPN-B. MUC5AC (gastric phenotype mucin) is frequently expressed in both BilIN lineages and IPN-B lineages in which foveolar (gastric) metaplasia is frequent. MUC2 is more commonly expressed in IPN-Bs and ICC with IPN-B while its expression is infrequent in BilINs and ICC with BilIN. Furthermore, in most cases of IPN-B, MUC2 was expressed in more than half of the neoplastic cells. Goblet cell (intestinal) metaplasia is frequently observed in IPN-B in which MUC2 expression (intestinal phenotype) is also frequent, whereas intestinal metaplasia is not frequent in BilIN lineage with no or infrequent MUC2 expression. These findings are compatible with the common occurrence of gastric metaplasia in both lineages, while intestinal metaplasia is more frequent in the IPN-B lineage.
Figure 3 MUC1 is expressed in neoplastic cells of BilIN-3 (B) but not in those of BilIN1 (A) (Immunostaining).
A: BilIN-1; B: BilIN-3.
As for the combined profile of MUC1 and MUC2 expression in each lesion, most IPN-Bs show the MUC1-/MUC2+ pattern[10,13,30]. Almost all colloid carcinomas exhibit the MUC1-/MUC2+ pattern. Tubular adenocarcinomas with IPN-B usually show MUC1+/MUC2+. These findings suggest that most non-invasive neoplastic lesions are MUC1-/MUC2+ during the carcinogenesis from IPN-B. When invasive ICC develops, a majority of tubular adenocarcinomas with IPN-B are MUC1-positive (MUC1+/MUC2+), whereas almost all colloid carcinomas retain a MUC1-negative pattern, such as MUC1-/MUC2+. In contrast, the MUC1-/MUC2- is most common in BilINs, and the frequency and degree of the MUC1+/MUC2- pattern increases stepwise in the BilIN lineage with a progression of histological grade (BilIN-1, BilIN-2/3 and ICC with BilIN). It is possible that the increased levels of MUC1 in BilIN and also IPN-B are associated with the development of tubular adenocarcinoma, while MUC1-negativity usually underlies the development of colloid carcinoma during the progression of ICC. Aberrant expression of MUC1 is reported to relate to the invasive and metastatic properties of carcinoma cells[13,30], which is consistent with the notion that colloid carcinoma negative for MUC1 has a better prognosis than tubular adenocarcinoma with BilIN or IPN-B with increased expression of MUC1.
Expression of CKs
In most cases of IPN-B or BilIN, CK7 (biliary cytokeratin) is expressed regardless of the degree of atypia, and is present in more than 10% of the tumor cells. CK20 (intestinal cytokeratin) expression is infrequent in BilINs and ICC with BilIN, while it is frequent and extensive in the IPN-B series[10]. Taken together, CK7+/CK20- is common in BilINs and ICC with BilIN, whereas CK7+/CK20+ is common in IPN-Bs and ICC with IPN-B. IPN-B with colloid carcinoma frequently shows the CK7+/CK20+ pattern.
EXPRESSION PATTERNS OF CANCER-RELATED MOLECULES IN BILIN ANDIPN-B
G1-S modulators (p21, p53, cyclin D1, and Dpc4)
Phase G1-S of the cell cycle is disrupted in the development of malignant neoplasms which is characterized by uncontrolled cell proliferation[31,32]. As modulators of G1-S, p16, p21, p53, cyclin D1 and the retinoblastoma (Rb) gene product are all important[31]. Cyclin D1 forms a complex with cyclin-dependent kinase (CDK), which phosphorylates Rb. Phosphorylated Rb allows cells to enter the S phase[33]. P16 and p21, both of which are CDK inhibitors, can prevent cell cycle progression at the G1-S checkpoint by binding to cyclin D1/CDK complexes[34]. P53 can up-regulate p21 expression under physiological conditions[35,36]. P53 is mutated in a large number of human malignant neoplasms, and mutated p53 is immunohistochemically detectable. Interestingly, these cell-cycle accelerants or decelerators are abnormally expressed not only in advanced malignant tumors, but also in non-invasive premalignant lesions. Transforming growth factor-β (TGF-β) has several biological functions, such as epithelial growth inhibition. TGF-β signaling is transmitted to the nucleus by cooperation with TGF-β receptors on cell membranes and intracytoplasmic mediators (Smads). Smad4/Dpc4 is one of the most important regulators involved in carcinogenesis. Inactivation of the DPC4 gene is frequently reported in some types of tumors, and a tumor-suppressive property is thus suggested[37,38].
In both the BilIN and IPNB series, the expression of p21, p53, and cyclin D1 is upregulated with histological progression, whereas the expression of Dpc4 is downregulated[23,39,40]. In BilIN, p21 expression is significantly upregulated already at BilIN-1 and also at BilIN-2 and -3. In contrast, levels of all of these molecules change both gradually, and stepwise, in IPNB lineages. Interestingly, p53 expression patterns differ significantly between BilIN and IPNB. That is, p53 expression is dramatically upregulated at the invasive stage of BilIN, while it is quite low in noninvasive BilIN. In contrast, p53 expression is already upregulated in IPN-B1 and reaches a plateau in IPN-B2 and invasive ICC. Taken together, p21, p53, cyclin D1, and Dpc4 are involved in the carcinogenesis of both BilIN and IPN-B, though their expression patterns are not the same during the progression of IPN-B to ICC and BilIN to invasive ICC, suggesting different pathways in terms of cancer-related gene expression, too[9,10,18,23,39,40].
Polycomb group protein EZH2 and p16INK4a
The p16INK4a gene displays its tumor-suppressive function by binding and inactivating cyclin-dependent kinases. Therefore, multiple epigenetic and genetic mechanisms for silencing or overcoming these tumor-suppressive processes are required for the development and progression of malignant tumors. During the progression of IPN-B in hepatolithiasis, p16INK4a expression is shown to be decreased stepwise with the progression through IPNB to invasive CC[23]. A methylation-specific polymerase chain reaction (MSP) showed that hypermethylation of the p16INK4a promoter was signficantly involved in this decrease in the expression of p16INK4a. Decreased expression of p16INK4a was seen in cases of IPN-B1 with mild dysplasia, and continued with the progression of IPN-B2 to ICC. MSP revealed that about a half of IPN-B foci harbored a hypermethylated p16INK4a promoter, and such foci were significantly correlated with decreased expression of p16INK4a protein. Cell proliferative activities exhibited a stepwise increase from IPN-B1 to IPN-B2. Together, p16INK4a’s inactivation, due mainly to its promoter hypermethylation, is a frequent and early event in cases of IPN-B, and may be related directly or indirectly to genetic and epigenetic alterations of other cell cycle regulators during the progression of IPN-B.
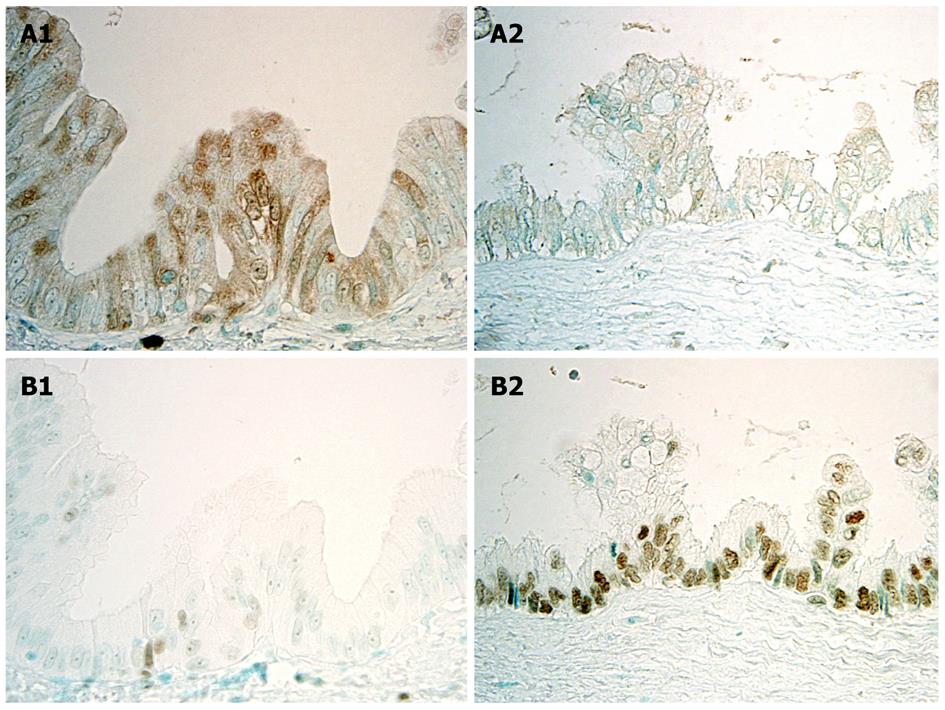
Recent studies showed that the Polycomb group proteins EZH2 and Bmi1 are chromatin-modifying enzymes, and interact with key elements that control cell growth and proliferation, such as the p16INK4a gene, and that abnormal expression of EZHE and Bmi1 is involved in tumorigenesis, including malignant transformation. Aberrant expression of EZH2 is regarded as a potential marker of advanced or aggressive cancer with a poor prognosis, differing from benign or indolent counterparts. As for the participation of EZH2 in multi-step cholangiocarcinogenesis with respect to the tumor suppressor gene p16INKa, it has been reported that the expression of p16INK4a, which was frequent in BilIN1, was decreased in BilIN-2/3 and invasive carcinoma, suggesting that a certain mechanism represses p16INKa expression with the progression of BilIN to invasive CC (Figures 4A and 5A)[24]. Interestingly, EZH2 expression showed a stepwise increase from BilIN-1, -2 and -3 to invasive carcinoma, suggesting that hypermethylation of the p16INK4a promoter was related to the aberrant expression of EZH2 (Figures 4B and 5B)[24]. The knockdown of EZH2 in cultured CC cells decreased p16INK4a methylation and decreased the binding of EZH2 to the p16INK4a promoter, suggesting that direct binding of EZH2 is involved in the regulation of the p16INK4a gene. It therefore seems conceivable that over-expression of EZH2 may induce hypermethylation of the p16INK4a promoter, followed by decreased expression of p16INK4a in the multi-step cholangiocarcinogenesis through the rhw BilIN lineage, particularly in cases of hepatolithiasis[22].
Figure 4 Immunohistochemical expression of p16INK4a and EZH2 in BilIN-1 and BilIN-2.
A: p16INK4a is intensely and frequently expressed in the cytoplasm and nuclei in BilIN-1 (left), but such expression is unclear in BilIN-2 (right); B: While EZH2 is occasionally expressed in the nuclei in BilIN-1 (left), this expression increases markedly in BilIN-2 (right)[22].
Figure 5 Semiquantitative evaluation of P16INK4a and EZH2 in BilIN-1, -2, and -3, and invasive cholangiocarcinoma (CC).
The labeling index (LI) reflects the percentage of cells immunohistochemically positive for P16INK4a or EZH2 in each lesion of BilIN-1, -2, and -3, and invasive CC. bP < 0.01 vs BilIN-1. A: The LI of P16INK4a expression (mean ± SD) decreases with the progressing of BilINs and is higher in invasive CC; B: Expression of EZH2 increases with the progression of BilINs and is greater in invasive CC[22].
Matrix metalloproteinases (MMPs)
MMPs are believed to play a pivotal role in the malignant behavior of cancer cells such as rapid tumor growth, invasion, and metastasis, by degrading the extracellular matrix (ECM)[41]. MMP-7 is the smallest MMP expressed exclusively in the tumor cells themselves. MMP-7 has proteolytic activity against components of the ECM such as collagens, proteoglycans, laminin, and fibronectin[42]. MMP-7 overexpression was observed in malignant tumors, including carcinomas of the colorectum and stomach[43]. MT1-MMP is expressed on the cancer cell surface as an invasion-promoting proteinase[44]. One of its major targets is type I collagen. MT1-MMP also regulates cell-ECM interaction by processing cell adhesion molecules, and eventually promotes cell migration as well. Recently, we showed that MMP-7 and MT1-MMP were commonly expressed in invasive ICC with BilIN, but not non-invasive lesions (BilIN-1, -2, -3), suggesting that the expression of MMP-7 and MT1-MMP was closely associated with invasive growth of the BilIN lineage[25]. In contrast, the IPNB lineage was only occasionally and weakly positive for these molecules, reflecting its favorable prognosis[3,45,46].
Cell adhesion molecules (β-catenin and E-cadherin)
A reduction of β-catenin in the cell membrane reflects a disruption of cell-to-cell adhesion, and E-cadherin is one subtype of transmembrane glycoprotein whose cytoplasmic domain is bound to α- and β-catenin. Interestingly, decreased membranous expression of β-catenin is correlated well with that of E-cadherin[25]. Decreased membranous expression of both these molecules is closely associated with the invasion of carcinoma cells, and is recognizable early on in both the BilIN and IPNB lineages. The membranous expression of β-catenin decreases with the progression of in both BilIN and IPN-B, particularly after the invasion and then metastasis of carcinoma cells. Membranous expression of β-catenin and E-cadherin decreased more markedly in ICC with BilIN than in ICC with IPNB, a finding compatible with the report of Sugimachi et al[47]. Interestingly, decreased membranous expression of E-cadherin and β-catenin in the BilIN and IPN-B lineages was associated with the aberrant expression of MMP-7 and MT1-MMP, suggesting that disruption of the membranous distribution of β-catenin and E-cadherin may result in conditions favorable for the invasion and metastasis of carcinoma cells of these two lineages which express MM-7 and MT1-MMP.
Cyclin D and c-myc
Cyclin D1 is known to bind to cyclin-dependent kinase (CDK) 2 or CDK 4, to act as a CDK inhibitor, and to inhibit Rb phosphorylation leading to an accelerated cell cycle progression and increased cell proliferation[48]. C-myc has mitogenic effects modulating regulators of cell cycle progression[49]. Overexpression of cyclin D1 was reported in about a half of ICCs and was associated with poor histological differentiation and a poor prognosis, and overexpression of c-myc was reported in about a half of ICCs[50,51]. As for precursor lesions of ICC, increased expression of cyclin D1 and c-myc was more frequent in the IPNB lineage than in the BilIN lineage, and was possibly related to the Wnt signaling pathway associated with the nuclear accumulation of β-catenin. Cyclin D1 and c-myc, targets of Wnt signalling, were frequently positive in the IPNB lineage, and interestingly, nuclear β-catenin staining was observed only in the IPNB lineage. Decreased membranous expression of β-catenin and E-cadherin is an early event in the tumorigenesis of both the BilIN and IPNB lineages[52]. The Wnt signaling pathway may therefore play an important role in the tumorigenesis of the IPNB lineage.