Copyright
©The Author(s) 2016.
World J Hepatol. Mar 18, 2016; 8(8): 395-400
Published online Mar 18, 2016. doi: 10.4254/wjh.v8.i8.395
Published online Mar 18, 2016. doi: 10.4254/wjh.v8.i8.395
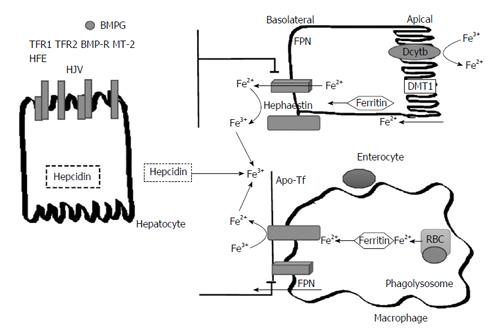
Figure 1 Pathways of Iron transport and metabolism.
The pathway of iron in enterocytes and macrophages as effected by hepcidin. Dietary non-heme iron is taken into the enterocyte via the DMT1. In order for iron to move across the brush border of the enterocyte via DMT1, it must first be reduced from Fe3+ to Fe2+ by DcytB. Once inside of the cell, iron can be sequestered into storage as ferritin or continue along the pathway into circulation. In this process, the iron exporter FPN located on the basolateral surface of enterocytes is responsible for the transport of ferrous iron into circulation. Once iron is in circulation, hephaestin oxidizes the ferrous iron back into the ferric state and then it immediately binds to plasma transferrin. The iron is now able to travel to sites of iron storage or where iron is required. In macrophages, phagolysosomes containing senescent RBC release iron which is also then exported into circulation via ferroportin. Hepcidin, a protein derived from the liver, regulates iron transport in the body by causing the internalization and degradation of FPN transporters on macrophages and the basolateral surface of enterocytes. Hepcidin is regulated based on body iron requirements by signals produced from the interaction between different proteins on hepatocytes. The interaction of the HFE protein and transferrin receptors 1 and 2 (TFR1 and TFR2) and the interaction between bone morphogenic protein (BMP6), hemojuvelin (HJV) and the bone morphogenic protein receptor (BMP-R) and matriptase 2 (MT-2). RBC: Red blood cells; FPN: Ferroportin; DMT1: Divalent metal-ion transporter 1; DcytB: Duodenal cytochrome B; HFE: Human factors engineering.
- Citation: Sivakumar M, Powell LW. Management of human factors engineering-associated hemochromatosis: A 2015 update. World J Hepatol 2016; 8(8): 395-400
- URL: https://www.wjgnet.com/1948-5182/full/v8/i8/395.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i8.395