Copyright
©The Author(s) 2016.
World J Hepatol. Jul 8, 2016; 8(19): 796-814
Published online Jul 8, 2016. doi: 10.4254/wjh.v8.i19.796
Published online Jul 8, 2016. doi: 10.4254/wjh.v8.i19.796
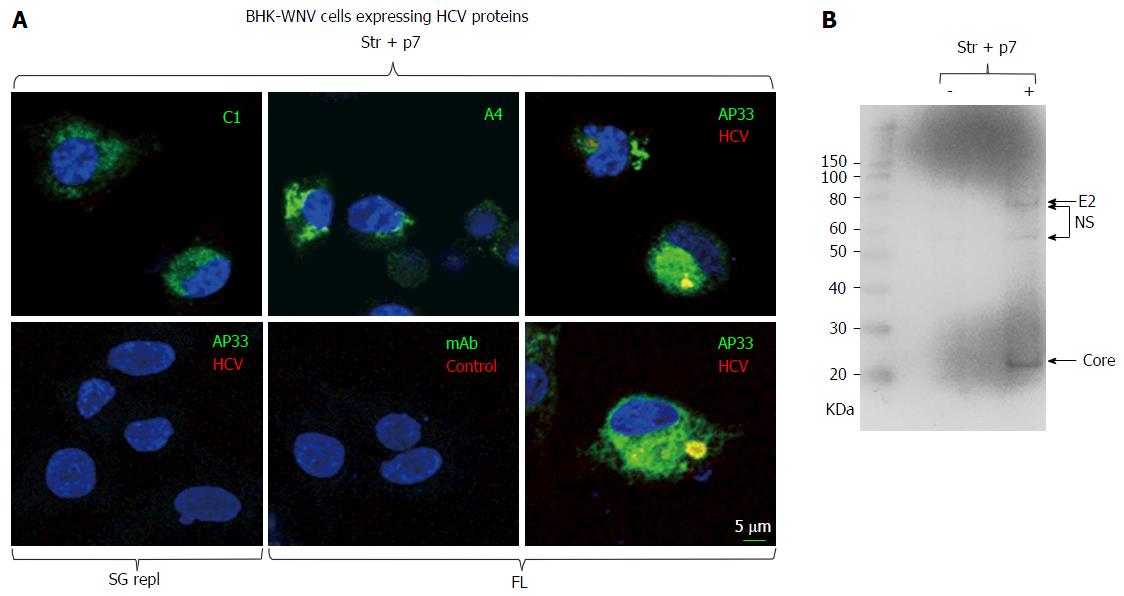
Figure 1 Immunodetection of hepatitis C virus proteins of genotype 1a expressed in baby hamster kidney-West Nile virus cells.
A: A plasmid encoding Str + p7 of HCV strain H77 (genotype 1a) from an early human cytomegalovirus promoter or a system of plasmids (P2B) expressing a subgenomic replicon or genome (FL) of same genotype in the cytoplasm (8) were transfected in BHK-WNV cells; after 2 d, IF study was performed with monoclonal antibodies (green) targeting core 9-21 (C1), envelope E1 (A4) and E2 (AP33) (28) glycoproteins of strain H77 (all IgG1a), or anti-rabbit IgG mouse Ab (mAb) of same isotype; and with human serum IgG (red) obtained from a patient recently cured from an infection of same genotype (HCV) or uninfected (control). Nuclei were counterstained with DAPI (blue) and cells were observed with a laser-scanning confocal microscope; B: BHK-WNV cells were either mock transfected (-) or transfected with a system of plasmids expressing the genome of H77 strain (+); after 3 d, cell lysates were prepared and human anti-HCV IgG tested in (a) were used as a Western blot probe. The scale on the left shows molecular weight markers. HCV: Hepatitis C virus; BHK-WNV: Baby hamster kidney-West Nile virus; Str + p7: Structural and p7 genes.
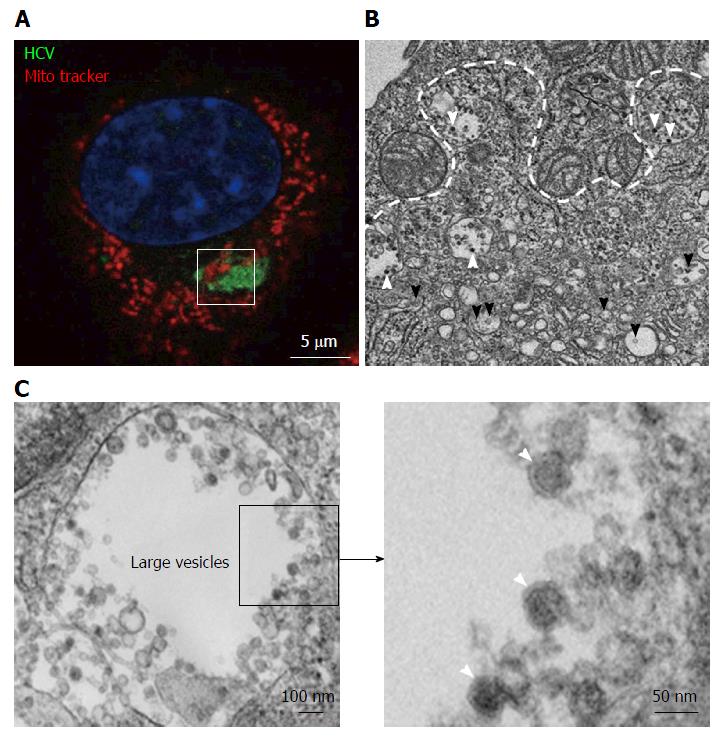
Figure 2 Transmission electron microscopy view of a hepatitis C virus compartment forming in baby hamster kidney-West Nile virus cells cells.
BHK-WNV cells were transfected with a mix of HCVbp-expressing and P2B plasmids (8). A: IF analysis showing a compartment recognized by human serum HCV IgG (green) surrounded by mitochondria (red); nucleus is counterstained with DAPI (blue). The white square delimits an area similar to that displayed in the next panel; B: Thin section observed by transmission electron microscopy: White arrowheads: Electron-dense viral particles in large vesicles; black arrowheads: Nascent viral particles in traffic vesicles; white dotted line: Limit of mitochondria surrounding the compartment containing viral particles (cf. area within white square in previous panel); C: Left panel: Example of large vesicle that develops in the cytoplasm of permissive BHK-WNV cells upon expression of full-length HCV genome; Right panel: Magnification of viral particles. HCV: Hepatitis C virus; BHK-WNV: Baby hamster kidney-West Nile virus.
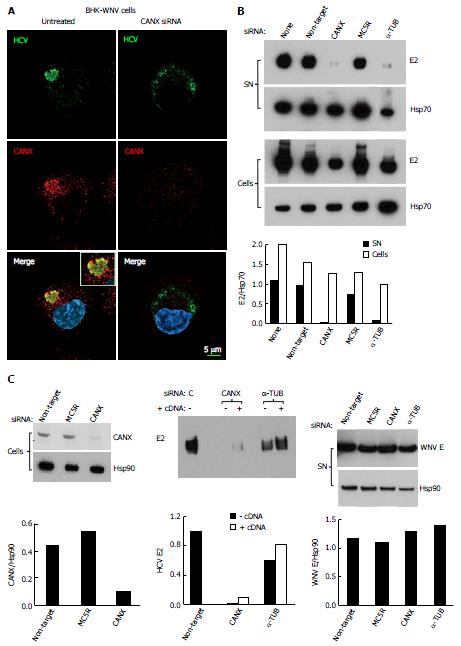
Figure 3 Involvement of calnexin and alpha-tubulin in the release of hepatitis C virus particles by baby hamster kidney-West Nile virus cells.
A: BHK-WNV cells were treated, or not, with CANX siRNA and, three days later, transfected with the HCV-coding and P2B plasmids. Two days later, IF was performed with anti-HCV serum of Figure 1 (green) and anti-CANX antibody (red) followed by confocal microcopy analysis; nucleus were counterstained with DAPI (blue); B: Top panels: BHK-WNV cells were treated with the indicated siRNA for 2 d, then transfected with a plasmid encoding full length HCV (HCVbp) in the cytoplasm. Contents in E2 envelope protein of both supernatant (SN) and cell lysate (Cells) were analyzed 2 d later by Western blot (WB); Bottom panel: Densitometry analysis; C: Left panels: BHK-WNV cells were treated with the indicated siRNA for 2 d and content in CANX was analyzed by WB; Hsp90 was used as a control; Middle panel: BHK-WNV cells were treated with siRNA targeting CANX or a-TUB transcript for 2 d; cells were then reseeded and transfected the next day with the HCV-coding plasmid together with a control plasmid (-) or one expressing the cDNA of the knocked-down transcript (+). Two days later, HCV materials released in SN were analyzed by WB; Right panels: BHK-WNV treated as in (B) were transfected with a plasmid encoding West Nile virus (WNV) structural genes (core, prM and E). Two days later, materials released in the SN were analyzed by WB with an antibody recognizing WNV E (29); Hsp90 was used as a control; Bottom panels: Densitometry analyses. CANX: Calnexin; α-TUB: Alpha-tubulin; HCV: Hepatitis C virus; BHK-WNV: Baby hamster kidney-West Nile virus; C: Control siRNA.
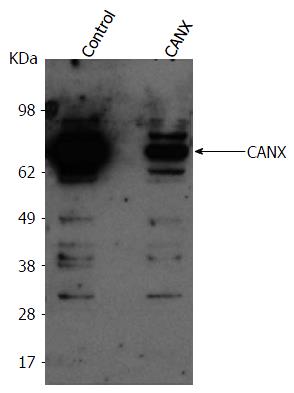
Figure 4 Baby hamster kidney-West Nile virus cells treated with the indicated siRNA (on top).
After 2 d, cells from both conditions were reseeded and transfected the next day with either a control plasmid or plasmid expressing CANX cDNA, respectively in control or CANX siRNA-treated BHK-WNV cells. The following day, content in CANX was analyzed by Western blot in cell lysates. CANX: Calnexin; BHK-WNV: Baby hamster kidney-West Nile virus.
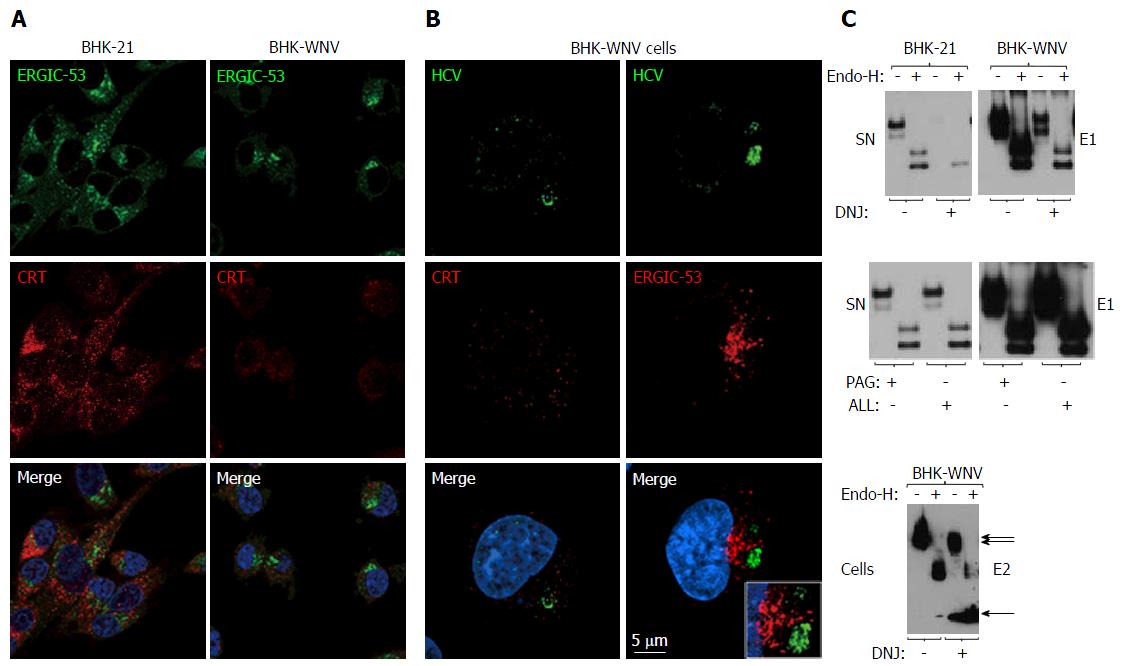
Figure 5 Calnexin and N-linked glycosylation are involved in the release of hepatitis C virus particles via a non-classical secretion path in baby hamster kidney-West Nile virus cells.
A: BHK-21 and BHK-WNV cells were transfected with the HCV expression plasmid system. IF was performed three days later with anti-ERGIC-53 (green) and anti-CRT (red) antibodies, followed by confocal microscopy analysis; B: Same protocol as in (A) with BHK-WNV cells transfected with the HCV-coding plasmids; C: Twelve hours after transfection with the HCV expression plasmid mix, parental BHK cells or BHK-WNV cells were treated, or not, with N- (DNJ) or O- (PAG, ALL) glycosylation inhibitors; materials released in SN (top and middle panels) or cell lysates (bottom panel) were collected, incubated with or without Endo-H and analyzed by Western blot. HCV: Hepatitis C virus; BHK-WNV: Baby hamster kidney-West Nile virus; ERGIC-53: Endoplasmic reticulum-Golgi intermediate compartment-protein of 53 kDa; CRT: Calreticulin; SN: Supernatants; DNJ: Deoxynojirimycin; Endo-H: Endo-β-N-acetylglucosaminidase H.
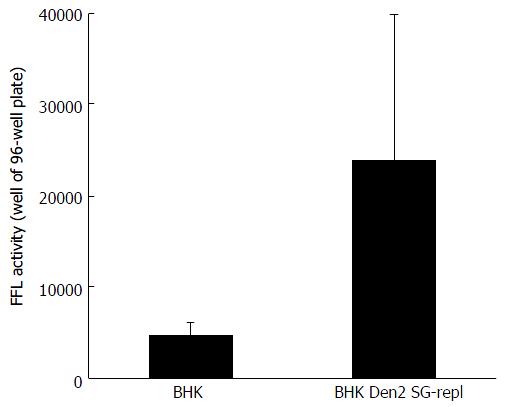
Figure 6 Huh-7.
5 cells were incubated with hepatitis C virus reporter particles particles produced either in parental baby hamster kidney cells or in baby hamster kidney cells chronically replicating a dengue 2 subgenomic replicon (similar to the West Nile virus’s). Infectivity was measured in target cells with a Firefly luciferase (FFL)-based reporter system, as previously decribed[8]. Error bars represent the SD in a representative experiment. BHK: Baby hamster kidney.
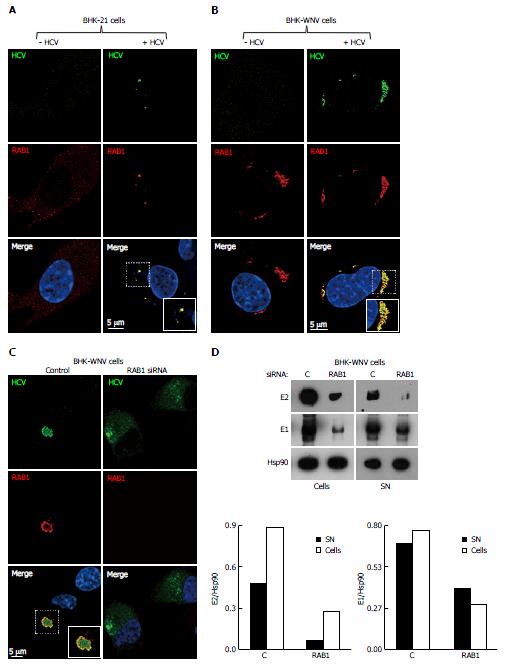
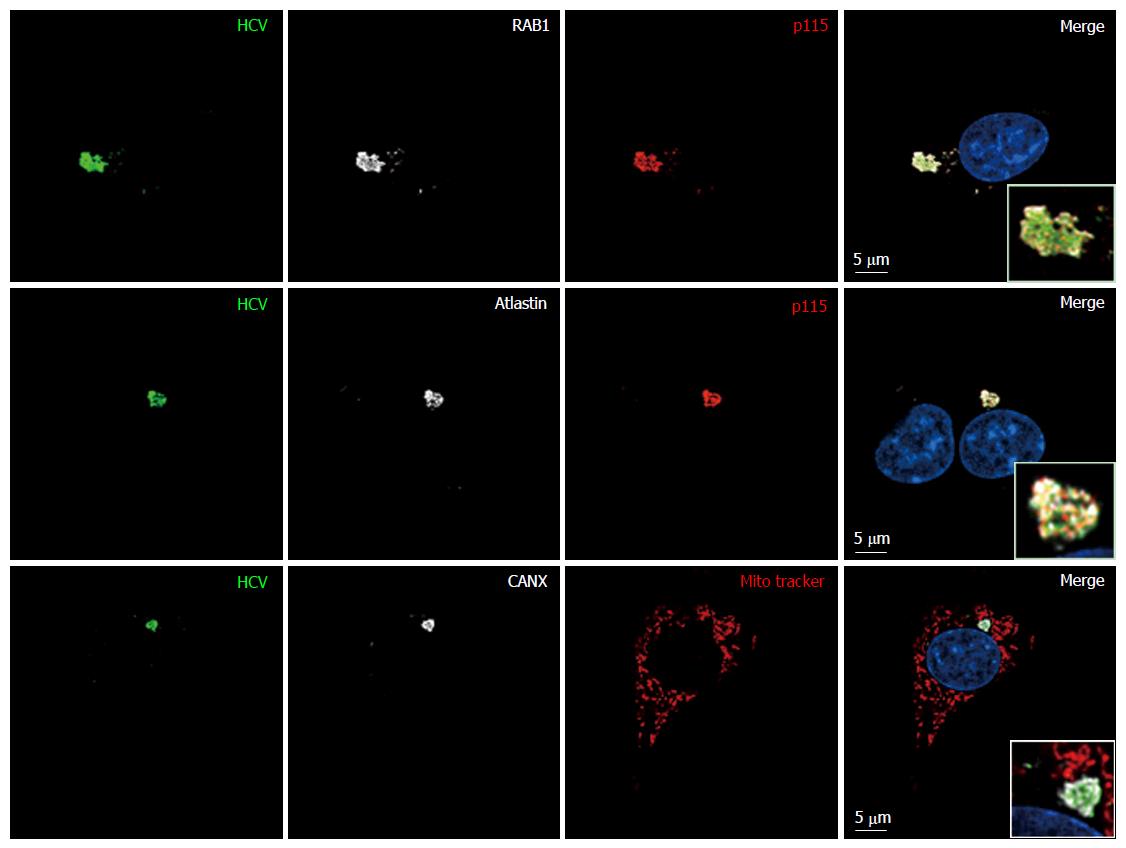
Figure 7 Release of hepatitis C virus particles by baby hamster kidney-West Nile virus cells requires RAB1 in a cytoplasmic subcompartment.
Three days after transfection with the HCV-coding and P2B plasmids, or not, IF of BHK-21 (A) or BHK-WNV (B) cells was performed with anti-HCV serum (green) and anti-RAB1 antibody (red), followed by confocal microscopy analysis; C: BHK-WNV cells treated (right panels) or not (left panels) with RAB1 siRNA were transfected with the HCV expression plasmid system; IF was performed as in (B); A-C: Nuclei were counterstained with DAPI (blue); D: BHK-WNV cells treated with RAB1 siRNA were transfected with the HCV expression plasmid system. Cells and SN were harvested 3 d later, and analyzed by Western blot; Hsp90 = control; bottom: Densitometry analysis. HCV: Hepatitis C virus; BHK-WNV: Baby hamster kidney-West Nile virus; SN: Supernatants.
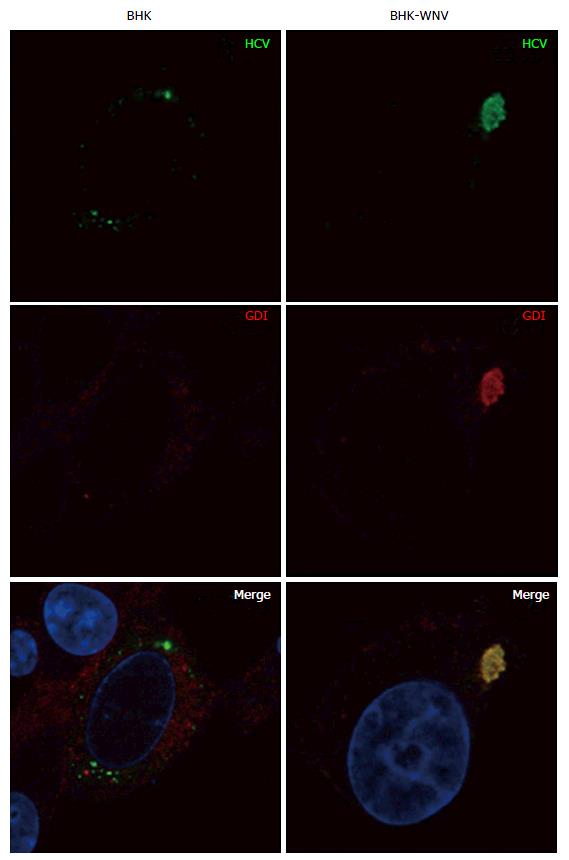
Figure 8 Parental or baby hamster kidney-West Nile virus cells were transfected to express hepatitis C virus genome in the cytoplasm and IF experiment was performed and analyzed as described throughout the manuscript.
HCV: Hepatitis C virus; BHK-WNV: Baby hamster kidney-West Nile virus; GDI: GDP dissociation inhibitor.
Figure 9 Parental or baby hamster kidney-West Nile virus cells were transfected to express hepatitis C virus genome in the cytoplasm and IF experiment was performed and analyzed as described throughout the manuscript.
HCV: Hepatitis C virus; BHK-WNV: Baby hamster kidney-West Nile virus.
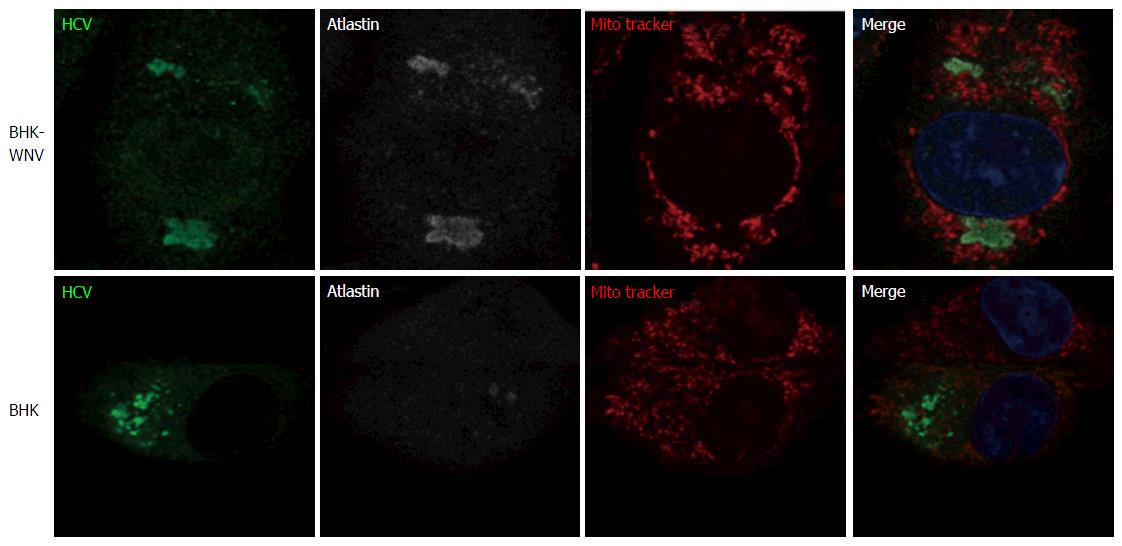
Figure 10 Endoplasmic reticulum-Golgi membrane remodelers (RAB1, p115 and atlastin) are recruited into the hepatitis C virus assembly compartment in baby hamster kidney-West Nile virus cells.
Three days after transfection of BHK-WNV cells with the HCV expression plasmid system, IF was performed with anti-HCV serum (green) and anti-p115 (red), RAB1, Atlastin-1 or CANX (white) antibodies. Mitochondria were labeled with Mito-Tracker-Orange-CMTMRos (red). HCV: Hepatitis C virus; BHK-WNV: Baby hamster kidney-West Nile virus; CANX: Calnexin.
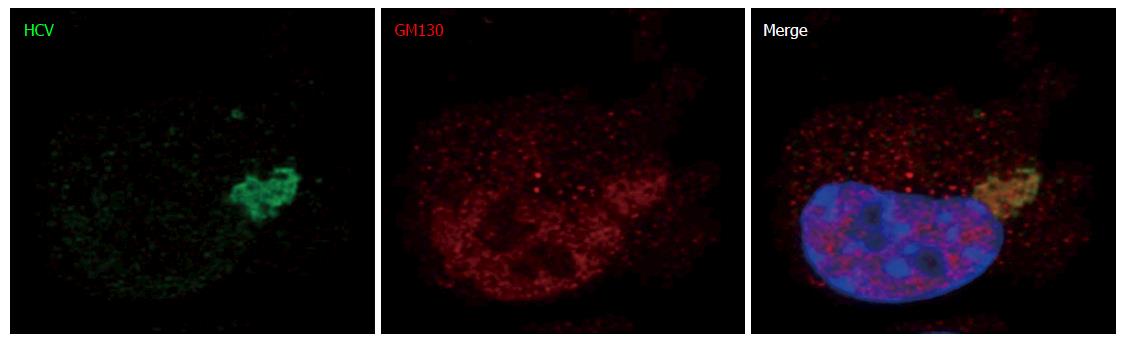
Figure 11 This experiment was performed with baby hamster kidney-West Nile virus cells transfected to express hepatitis C virus genome in the cytoplasm and IF experiment was performed and analyzed as described throughout the manuscript (anti-GM130 monoclonal antibodies have been reported to cross-react with an unidentified protein of lower molecular weight).
HCV: Hepatitis C virus.
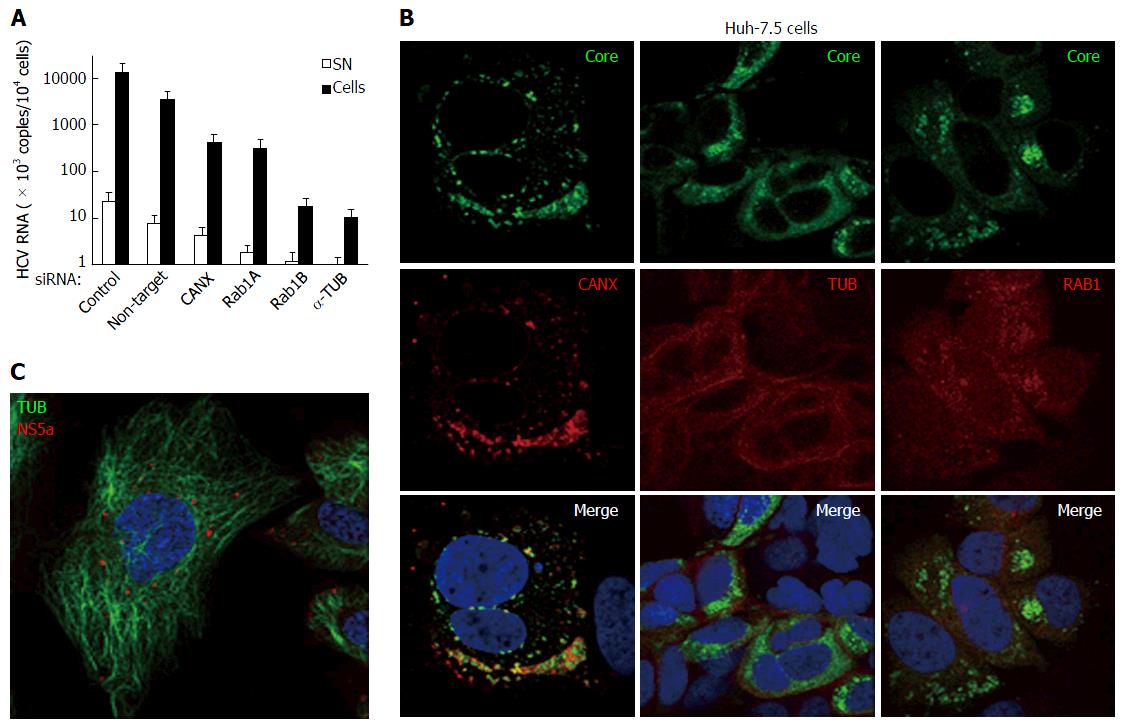
Figure 12 Hepatitis C virus produced in cultured human hepatic cells involves same cellular factors as those enhancing hepatitis C virus production in baby hamster kidney-West Nile virus cells.
A: Huh-7.5 cells were seeded on 24-well plates and the next day were transfected, or not, with siRNA, as indicated. After 2 d, cells were reseeded into 24-well plates and, the next day, incubated at a MOI = 0.5 with HCVcc produced with the JFH-1 strain in Huh-7.5 cells. At 3 dpi, HCV RNA contents were determined in the cells (closed bars) and SN (open bars) by RT-qPCR (TaqMan). Results are plotted on a log-scale; errors bars represent maximum variations observed in this assay; B: Huh-7.5 cells were electroporated with in vitro-transcribed genome of the JFH-1 strain and passaged for two weeks, then were seeded onto coverslips. Two days later, IF was performed with anti-HCV core 7-50 (green) and anti-human CANX, tubulin (TUB) or RAB1 antibodies (red); C: Huh-7.5 cells were inoculated with HCVbp-4cys produced in permissive BHK-WNV cells (8); 2 d later, the cells were incubated with both ReASH (red) and Taxol fluorophore conjugate (green); result representative of two independent experiments; B and C: Nuclei were counterstained with DAPI and cells were observed by confocal microscopy. CANX: Calnexin; HCV: Hepatitis C virus; BHK-WNV: Baby hamster kidney-West Nile virus; SN: Supernatants.
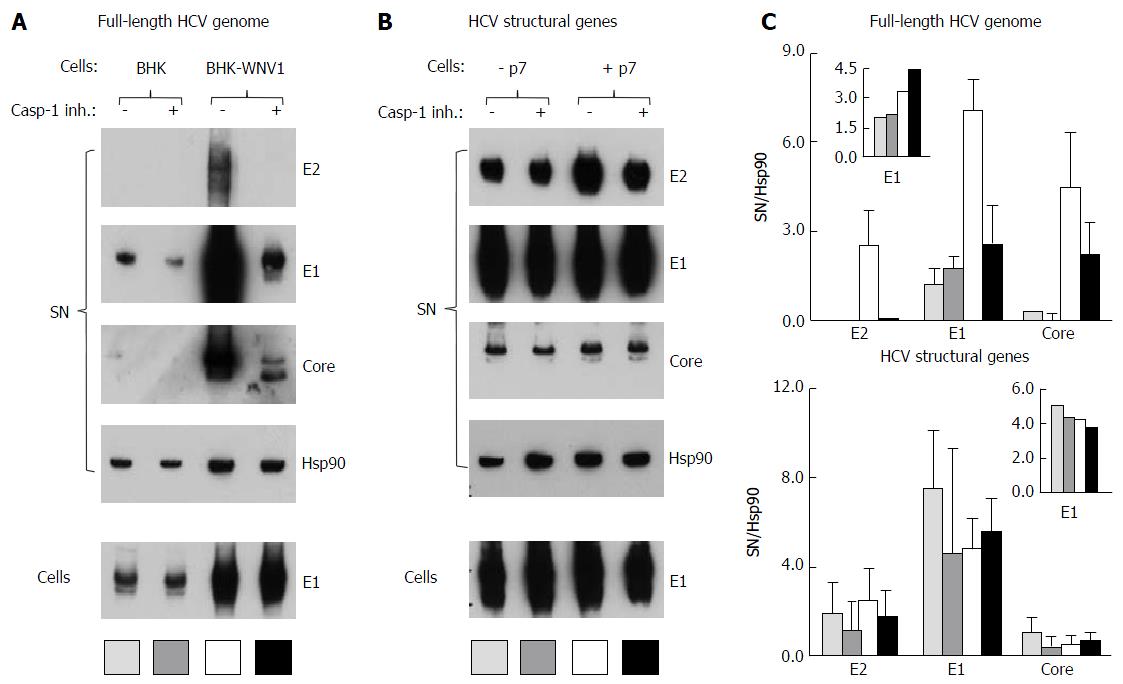
Figure 13 Caspase-1 inhibitor conditionally inhibits the secretion of hepatitis C virus particles by baby hamster kidney-West Nile virus cells.
A: BHK-21 and BHK-WNV cells were transfected with a mix of HCVbp-coding and P2B plasmids; the next day, a caspase-1 inhibitor was added in the culture medium and the cells were incubated for 2 more days; cell lysates and HCV particles were harvested and analyzed by Western blot (WB); B: BHK-WNV cells were transfected with a plasmid coding for the structural (core, E1, E2) genes of HCV H77 strain, plus (+) or minus (-) p7, then were analyzed as in (A); C: Quantification of WBs: Top panels for (A) and bottom panels for (B); bar inside patterns are displayed underneath corresponding data; error bars represent standard deviations; inserts = results in cells. HCV: Hepatitis C virus; BHK-WNV: Baby hamster kidney-West Nile virus.
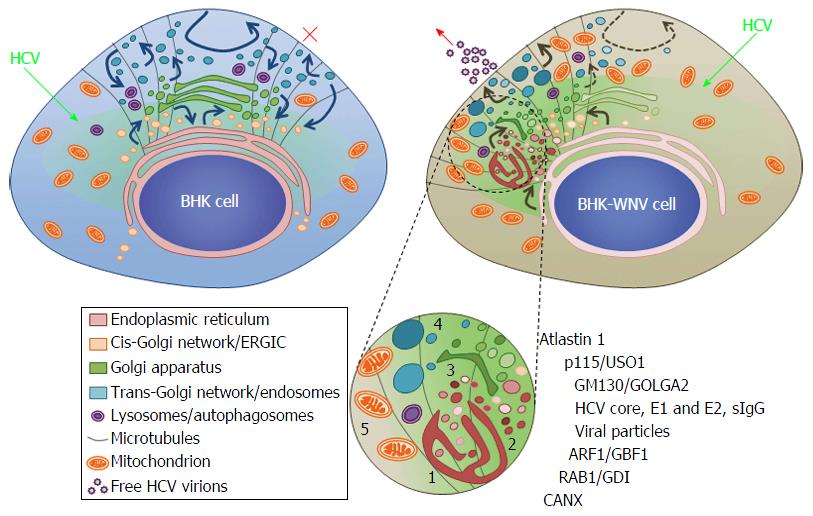
Figure 14 Model of a hepatitis C virus assembly compartment in baby hamster kidney-West Nile virus cells.
Schematic organization of cell traffic in parental BHK-21 (top left) and BHK-WNV (top right) cells; curved arrows represent cell traffic; HCV genome is produced by the P2B plasmid system (green arrows) and expressed (greenish areas) in the cytoplasm of BHK cells. Bottom, sketches’ legend and close up of a HCV assembly site (cf. also Figure 2B): (1) convoluted membranes; (2) vesicular packets; (3) Golgi cisternæ; (4) large vesicles filled with viral particles; and (5) mitochondrion; host and viral factors identified within this compartment. SIgG: Antibodies from the serum of a cured HCV patient; CANX: Calnexin; HCV: Hepatitis C virus; BHK-WNV: Baby hamster kidney-West Nile virus; ERGIC: Endoplasmic reticulum-Golgi intermediate compartment; GDI: GDP dissociation inhibitor.
- Citation: Triyatni M, Berger EA, Saunier B. Assembly and release of infectious hepatitis C virus involving unusual organization of the secretory pathway. World J Hepatol 2016; 8(19): 796-814
- URL: https://www.wjgnet.com/1948-5182/full/v8/i19/796.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i19.796