Copyright
©The Author(s) 2015.
World J Hepatol. Aug 18, 2015; 7(17): 2058-2068
Published online Aug 18, 2015. doi: 10.4254/wjh.v7.i17.2058
Published online Aug 18, 2015. doi: 10.4254/wjh.v7.i17.2058
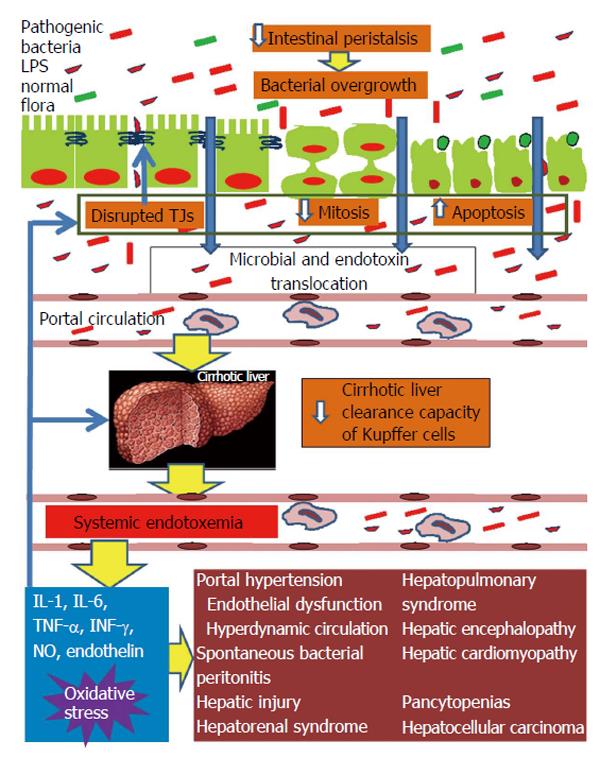
Figure 1 Pathophysiological overview of intestinal barrier dysfunction in liver cirrhosis and its interconnection with cirrhosis’ complications from diverse organs.
Liver cirrhosis delays intestinal motility thus changing the indigenous gut microecology and promoting intraluminal bacterial and endotoxin overgrowth. In parallel, the structural and functional integrity of the intestinal mucosa is disrupted leading to increased gut permeability. Important factors implicated in increased intestinal permeability are the disruption of the tight junctions structural complex and altered epithelial homeostasis, with decreased mitotic activity and increased apoptosis of enterocytes. Systemic cytokinemia and oxidative stress are pivotal promoters of these intestinal alterations. Increased gut permeability permits the escape of intraluminal bacteria and endotoxins initially into portal blood and subsequently, through a decreased clearance capacity of the cirrhotic liver, into systemic circulation. Systemic endotoxemia activates a systemic inflammatory response with release of interleukin-1 (IL-1), IL-6, tumor necrosis factor-alpha, interferon-γ, nitric oxide and endothelin-1, which can induce circulatory and remote organ dysfunction, partially through promotion of reactive oxygen species formation in the endothelium, lung, kidney, brain, heart and bone marrow. At the same time, the endotoxin-induced increased systemic levels of proinflammatory cytokines and oxidative stress aggravate intestinal and hepatic injury, further promoting bacterial translocation and endotoxemia, thus, maintaining the vicious cycle of gut barrier dysfunction, bacterial and endotoxin translocation, systemic release of proinflammatory cytokines and oxidative stress, complications of cirrhosis from diverse organs. TJ: Tight junction; LPS: Lipopolysaccharide.
- Citation: Tsiaoussis GI, Assimakopoulos SF, Tsamandas AC, Triantos CK, Thomopoulos KC. Intestinal barrier dysfunction in cirrhosis: Current concepts in pathophysiology and clinical implications. World J Hepatol 2015; 7(17): 2058-2068
- URL: https://www.wjgnet.com/1948-5182/full/v7/i17/2058.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i17.2058