Copyright
©The Author(s) 2015.
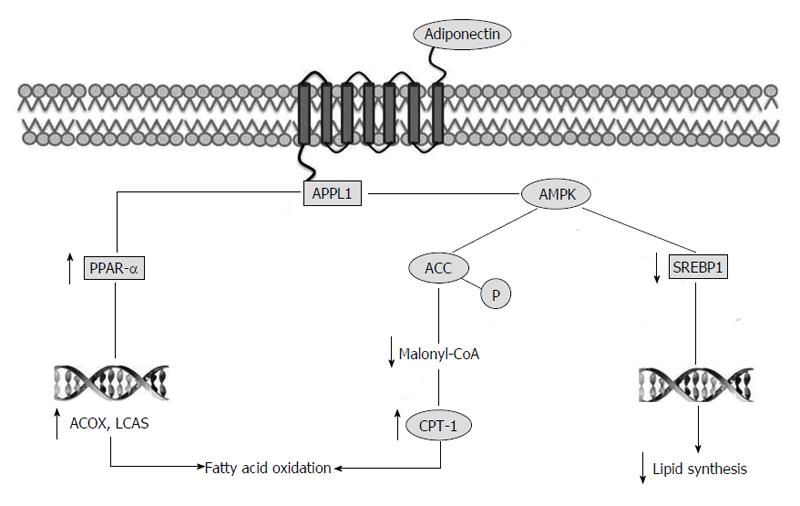
Figure 1 Molecular pathways involved in anti-steatotic effect of adiponectin.
The interaction between adiponectin receptor and APPL1 causes activation of AMPK. AMPK inhibits ACC by phosphorylation. Inhibition of ACC increases fatty acid oxidation by blocking the production of malonyl-CoA, the allosteric inhibitor of carnitine palmitoyl transferase 1. AMPK downregulates the expression of SREBP1c, a transcription factor that regulates different genes involved in lipid synthesis. Finally, APPL1 stimulates PPAR-α, another transcriptional factor controlling genes involved in fat oxidation. APPL1: Phosphotyrosine interaction, PH domain and leucine zipper containing 1; AMPK: AMP-activated protein kinase; ACC: Acetyl-CoA carboxylase; SREBP1: Sterol regulatory element-binding protein 1; PPAR-α: Peroxisome proliferator-activated receptor alpha; CPT-1: Carnitine palmitoyl transferase 1; ACOX: Acyl-CoA oxidase; LCAS: Long chain acyl-CoA synthetase.
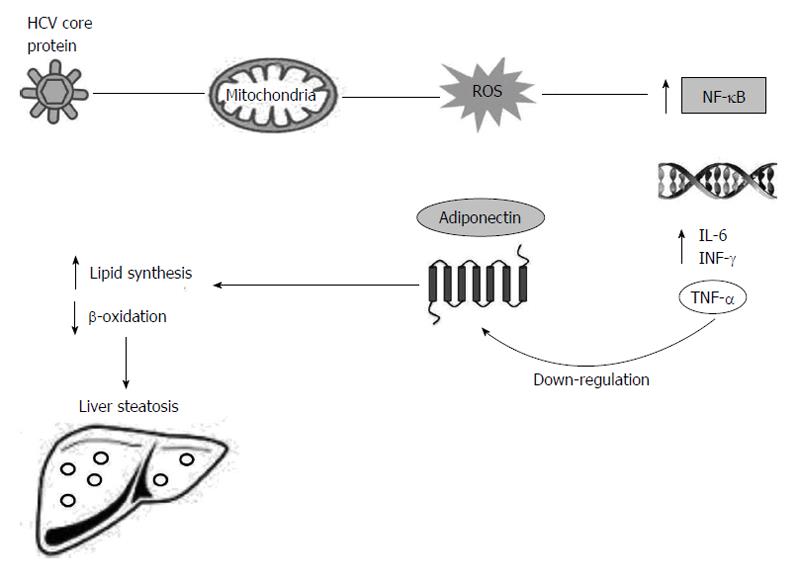
Figure 2 Liver steatosis induced by down-regulation of adiponectin and its receptor in chronic hepatitis C virus infection.
HCV core protein associated with the mitochondria leads to increased ROS that activates NF-κB. As a consequence of NF-κB activation, expression of a variety of cytokines is increased, including TNF-α, IL-6 and INF-γ. TNF-α modulates adipocytes and induces reduction in the production of adiponectin and its receptor. Reduced levels of adiponectin induce the increase in the synthesis of free fatty acids and reduce β-oxidation, causing liver steatosis in the HCV chronic infected patients. HCV: Hepatitis C virus; NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells; IL: Interleukin; IFN-γ: Interferon-γ; TNF-α: Tumour necrosis factor α; ROS: Reactive oxygen species.
- Citation: Peta V, Torti C, Milic N, Focà A, Abenavoli L. Adiponectin serum level in chronic hepatitis C infection and therapeutic profile. World J Hepatol 2015; 7(1): 44-52
- URL: https://www.wjgnet.com/1948-5182/full/v7/i1/44.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i1.44