Copyright
©2014 Baishideng Publishing Group Inc.
World J Hepatol. Dec 27, 2014; 6(12): 880-893
Published online Dec 27, 2014. doi: 10.4254/wjh.v6.i12.880
Published online Dec 27, 2014. doi: 10.4254/wjh.v6.i12.880
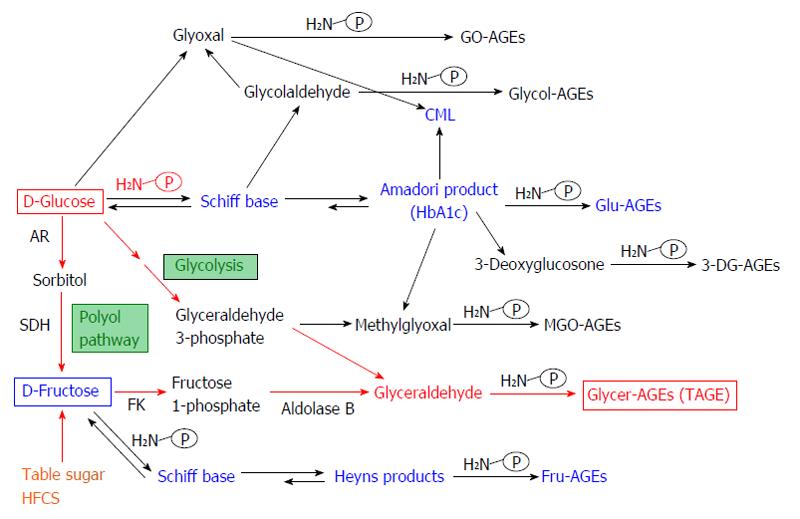
Figure 1 Alternative in vivo advanced glycation end-product synthesis routes.
Reducing sugars, such as glucose, fructose and glyceraldehyde, are known to react non-enzymatically with the amino groups of proteins to form reversible Schiff bases and Amadori product/Heyns products. These early glycation products undergo further complex reactions such as rearrangement, dehydration, and condensation to become irreversibly cross-linked, heterogeneous fluorescent derivatives termed advanced glycation end-products (AGEs). Glu-AGEs: Glucose-derived AGEs; Fru-AGEs: Fructose-derived AGEs; Glycer-AGEs: Glyceraldehyde-derived AGEs; Glycol-AGEs: Glycolaldehyde-derived AGEs; MGO-AGEs: Methylglyoxal-derived AGEs; GO-AGEs: Glyoxal-derived AGEs; 3-DG-AGEs: 3-deoxyglucosone-derived AGEs; CML: N-(carboxymethyl)lysine; P-NH2: A free amino residue; HbA1c: Hemoglobin A1c; TAGE: Toxic AGEs; HFCS: High-fructose corn syrup; AR: Aldose reductase; SDH: Sorbitol dehydrogenase; FK: Fructokinase.
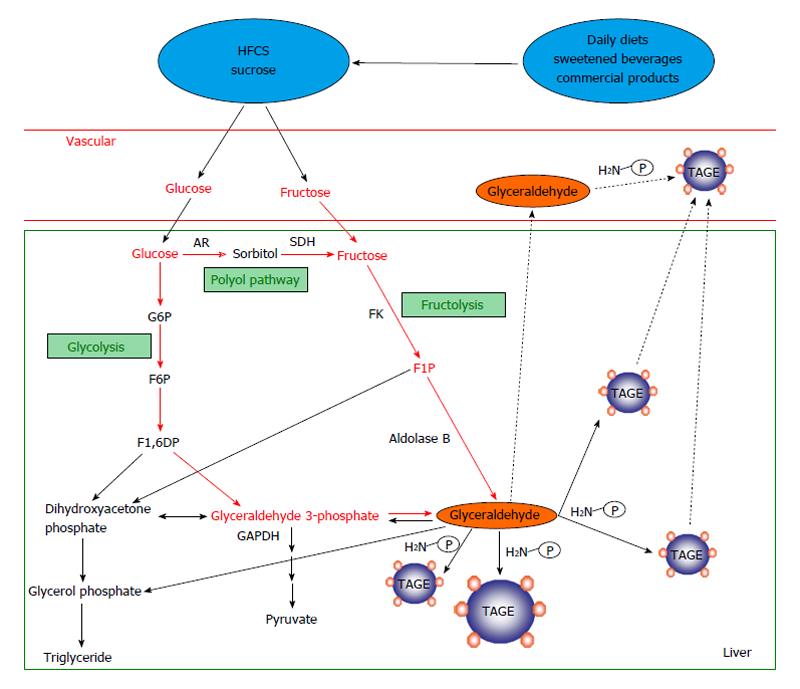
Figure 2 Routes for in vivo glyceraldehyde-derived advanced glycation end-products synthesis.
The glycolytic intermediate glyceraldehyde 3-phosphate (G3P) is usually catabolized (glycolysis) by the enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH). However, reductions in GAPDH activity lead to the intracellular accumulation of G3P. As a result, G3P metabolism starts to occur via an alternative pathway, leading to a rise in the concentration of glyceraldehyde, which promotes the synthesis of TAGE. Fructokinase phosphorylates fructose to fructose 1-phosphate, which is then broken down into dihydroxyacetone phosphate and glyceraldehyde by aldolase B (fructolysis). The resultant glyceraldehyde is transported (or leaks passively) across the cell membrane. Glyceraldehyde promotes the formation of TAGE both intracellularly and extracellularly. AGEs: Advanced glycation end-products; TAGE: Toxic AGEs (glyceraldehyde-derived AGEs); HFCS: High-fructose corn syrup; AR: Aldose reductase; SDH: Sorbitol dehydrogenase; FK: Fructokinase; G6P: Glucose 6-phosphate; F6P: Fructose 6-phosphate; F1,6DP: Fructose 1,6-diphosphate; F1P: Fructose 1-phosphate; P-NH2: Free amino residue.
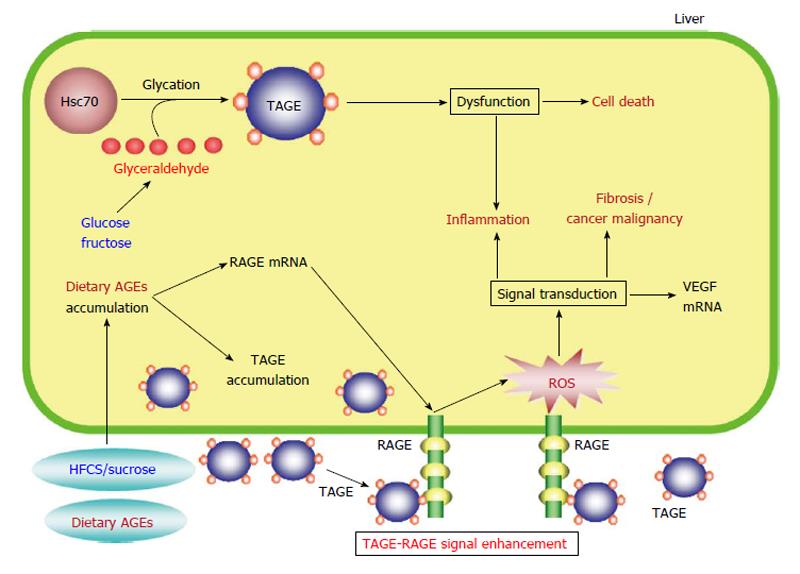
Figure 3 Proposed model for toxic advanced glycation end-products-mediated responses in the liver.
HFCS/sucrose and dietary AGEs, which are normally found in sweetened beverages and commercial food products, are taken into the body, where they enhance the production/accumulation of TAGE, upregulate RAGE mRNA expression, and increase serum TAGE concentrations, leading to TAGE-RAGE interactions. The interaction between TAGE and RAGE alters intracellular signaling, gene expression, and the release of pro-inflammatory molecules and also induces oxidative stress in hepatocytes and hepatic stellate cells, which might contribute to the pathological changes observed in NAFLD/NASH. The formation of intracellular TAGE is associated with protein dysfunction followed by inflammation and cell death. Extracellular TAGE induce inflammation and fibrosis/cancer malignancy via RAGE signaling. AGEs: Advanced glycation end-products; TAGE: Toxic AGEs; RAGE: Receptor for AGEs; Hsc70: Heat shock cognate 70; ROS: Reactive oxygen species; VEGF: Vascular endothelial growth factor; HFCS: High-fructose corn syrup; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis.
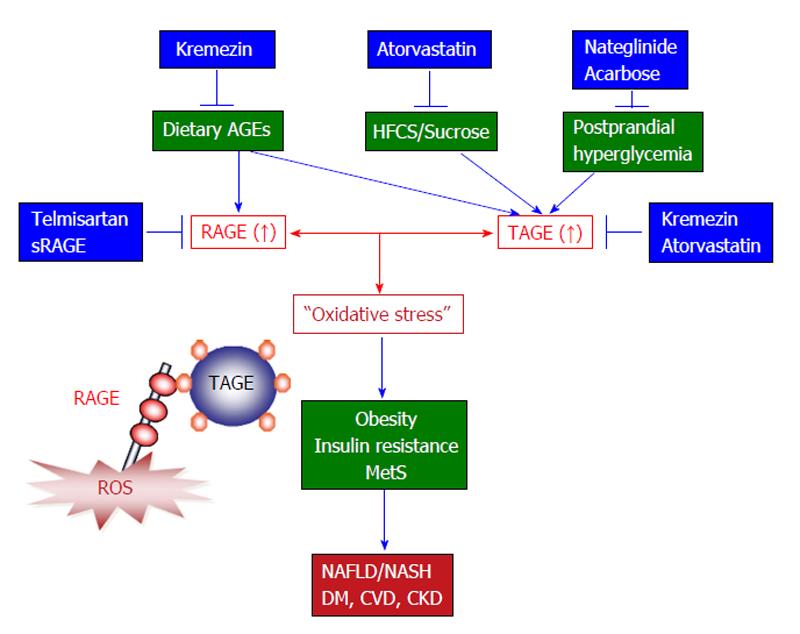
Figure 4 The toxic advanced glycation end-products-receptor for advanced glycation end-products system and novel treatments that target this system to prevent the development and/or progression of non-alcoholic steatohepatitis.
Accumulating evidence suggests that TAGE-RAGE interactions affect intracellular signaling, gene expression, and the release of pro-inflammatory molecules and also induce oxidative stress in numerous types of cells, all of which have the potential to contribute to the pathological changes associated with lifestyle-related diseases including NAFLD/NASH. Since TAGE display the strongest binding affinities for RAGE and have adverse effects on diabetic vessels through their interactions with RAGE, TAGE might be partly responsible for the increased risk of cardiovascular disease (CVD) seen in diabetes mellitus (DM) patients and the impaired glucose tolerance observed in patients with postprandial hyperglycemia. NAFLD is considered to be a hepatic symptom of metabolic syndrome (MetS) and is strongly associated with insulin resistance, obesity, and abnormalities in glucose and lipid metabolism. It is important to consider the amounts HFCS/sucrose and AGEs present in foods to prevent liver disease, particularly in individuals that are at high risk of developing NAFLD/NASH, DM, CVD, or chronic kidney disease (CKD). Taken together, the present study suggests that TAGE could be used as novel therapeutic targets for the prevention of lifestyle-related diseases. Therefore, inhibiting the formation of TAGE, blocking TAGE-RAGE interactions, and suppressing the expression of RAGE or its downstream effectors all have potential as therapeutic strategies against lifestyle-related disease including NAFLD/NASH. AGEs: Advanced glycation end-products; TAGE: Toxic AGEs; RAGE: Receptor for AGEs; sRAGE: Soluble form of RAGE; NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; HFCS: High-fructose corn syrup.
- Citation: Takeuchi M, Takino JI, Sakasai-Sakai A, Takata T, Ueda T, Tsutsumi M, Hyogo H, Yamagishi SI. Involvement of the TAGE-RAGE system in non-alcoholic steatohepatitis: Novel treatment strategies. World J Hepatol 2014; 6(12): 880-893
- URL: https://www.wjgnet.com/1948-5182/full/v6/i12/880.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i12.880