Copyright
©2013 Baishideng Publishing Group Co.
World J Hepatol. Mar 27, 2013; 5(3): 152-155
Published online Mar 27, 2013. doi: 10.4254/wjh.v5.i3.152
Published online Mar 27, 2013. doi: 10.4254/wjh.v5.i3.152
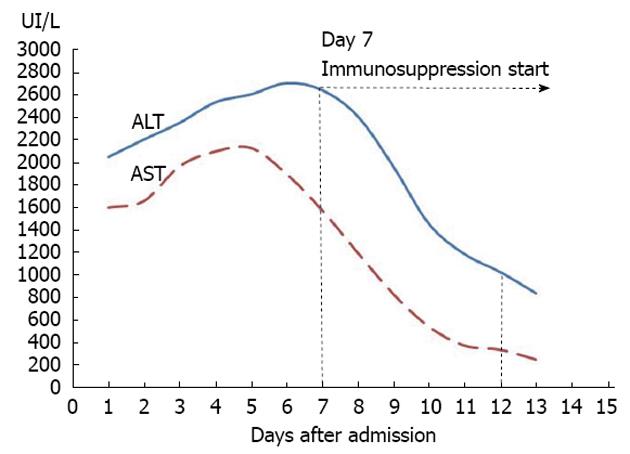
Figure 1 Evolution of aspartate aminotransferase and alanine aminotransferase during the hospitalization period.
Introduction of immunosuppression was as shown in the figure at day 7, when aspartate aminotransferase (AST) levels were already lowering. ALT: Alanine aminotransferase.
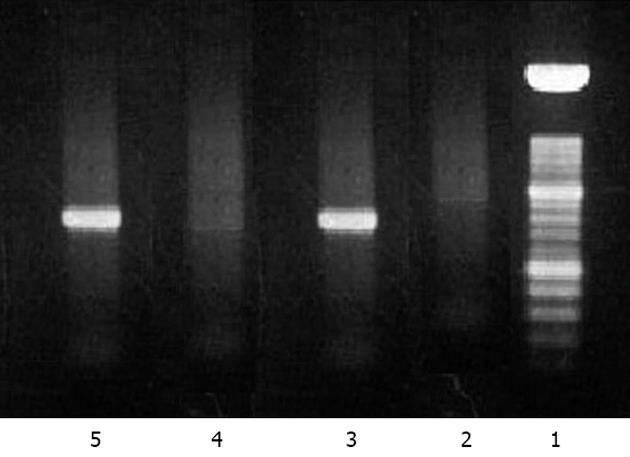
Figure 2 Electrophoresis with agarose gel 2%.
Reverse transcription polymerase chain reaction (RT-PCR) for hepatitis E virus (HEV) RNA from the liver specimen was performed using a First Strand cDNA synthesis kit for RT-PCR from Roche Diagnostics (Indianapolis, United States) according to the manufacturer’s instructions. This figure demonstrates the positive detection of HEV RNA with compatible strands 5 and 3. 1: Marker 100 bp (Promega); 2: White amplification; 3: Positive control for HEV RNA; 4: Negative control for HEV RNA; 5: Liver biopsy sample (HT370).
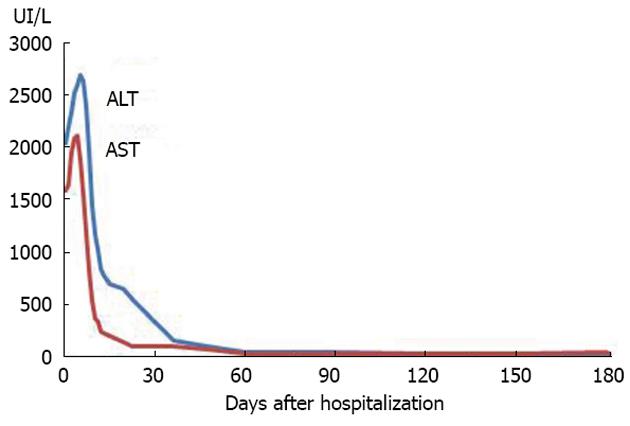
Figure 3 Evolution of aspartate aminotransferase and alanine aminotransferase until the 6th month after the hospitalization, with normalization soon after day 30, under no immunosuppression.
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase.
- Citation: Vieira CL, Baldaia C, Fatela N, Ramalho F, Cardoso C. Case of acute hepatitis E with concomitant signs of autoimmunity. World J Hepatol 2013; 5(3): 152-155
- URL: https://www.wjgnet.com/1948-5182/full/v5/i3/152.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i3.152