Copyright
©2010 Baishideng.
World J Hepatol. Jun 27, 2010; 2(6): 226-232
Published online Jun 27, 2010. doi: 10.4254/wjh.v2.i6.226
Published online Jun 27, 2010. doi: 10.4254/wjh.v2.i6.226
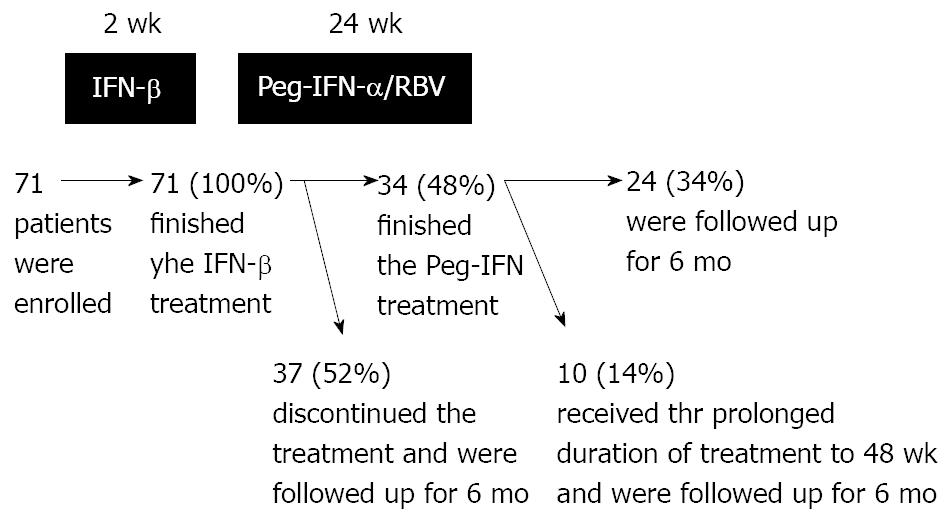
Figure 1 Duration of interferon-β (IFN-β) therapy and peginterferon-α (Peg-IFN-α) plus ribavirin (RBV) combination therapy, and the numbers of patients.
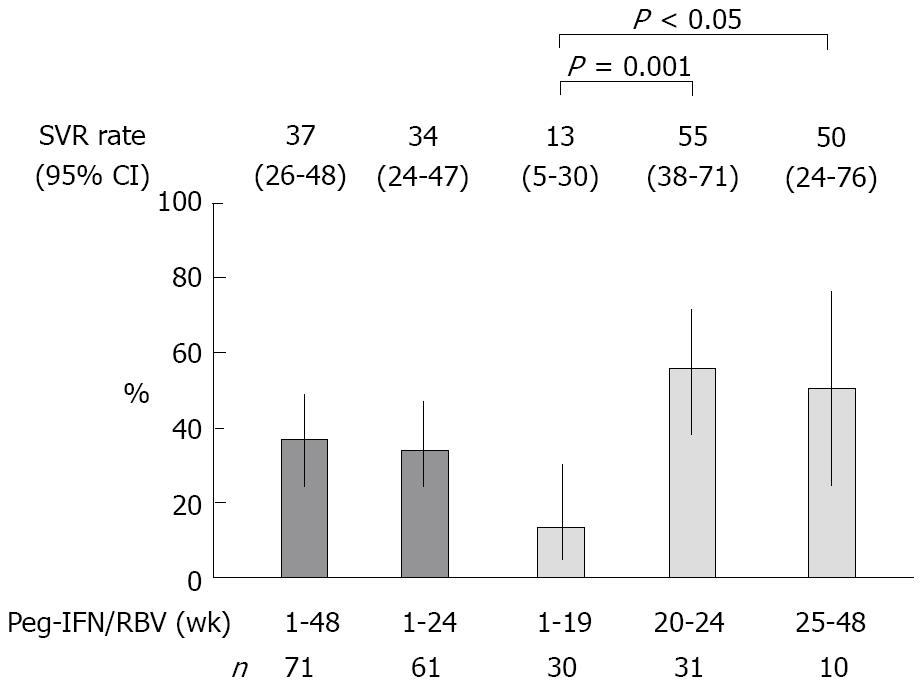
Figure 2 The SVR rate and 95% confidence interval (95% CI) by dosing duration of peginterferon (Peg-IFN) plus ribavirin (RBV) after interferon-β induction therapy.
The significance of the difference was evaluated employing Fisher’s exact test.
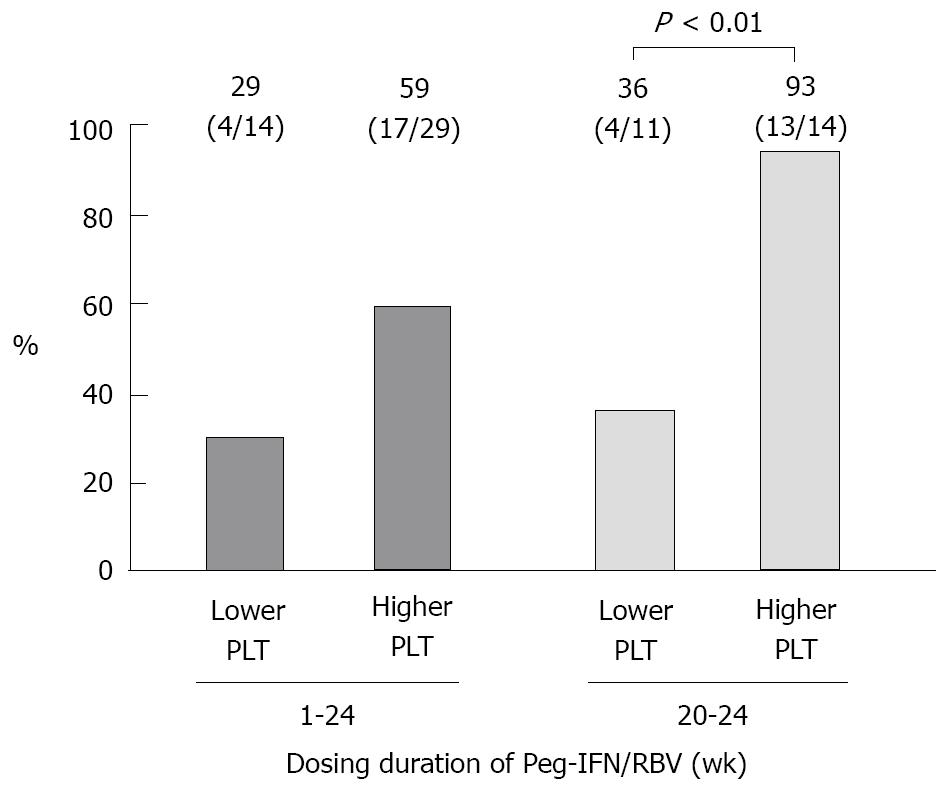
Figure 3 The SVR rate (%, n) by the baseline platelet count in patients whose HCV-RNA level decreased to below 3.
3 Log IU/mL at the end of interferon-β treatment. Higher PLT indicates a platelet (PLT) count of ≥ 130 000/mL at the start of therapy. The significance of the difference was evaluated using Fisher’s exact test. Peg-IFN: Peginterferon; RBV: Ribavirin.
- Citation: Okushin H, Morii K, Uesaka K, Yuasa S. Twenty four-week peginterferon plus ribavirin after interferon-β induction for genotype 1b chronic hepatitis C. World J Hepatol 2010; 2(6): 226-232
- URL: https://www.wjgnet.com/1948-5182/full/v2/i6/226.htm
- DOI: https://dx.doi.org/10.4254/wjh.v2.i6.226