Copyright
©The Author(s) 2020.
World J Hepatol. Jul 27, 2020; 12(7): 332-349
Published online Jul 27, 2020. doi: 10.4254/wjh.v12.i7.332
Published online Jul 27, 2020. doi: 10.4254/wjh.v12.i7.332
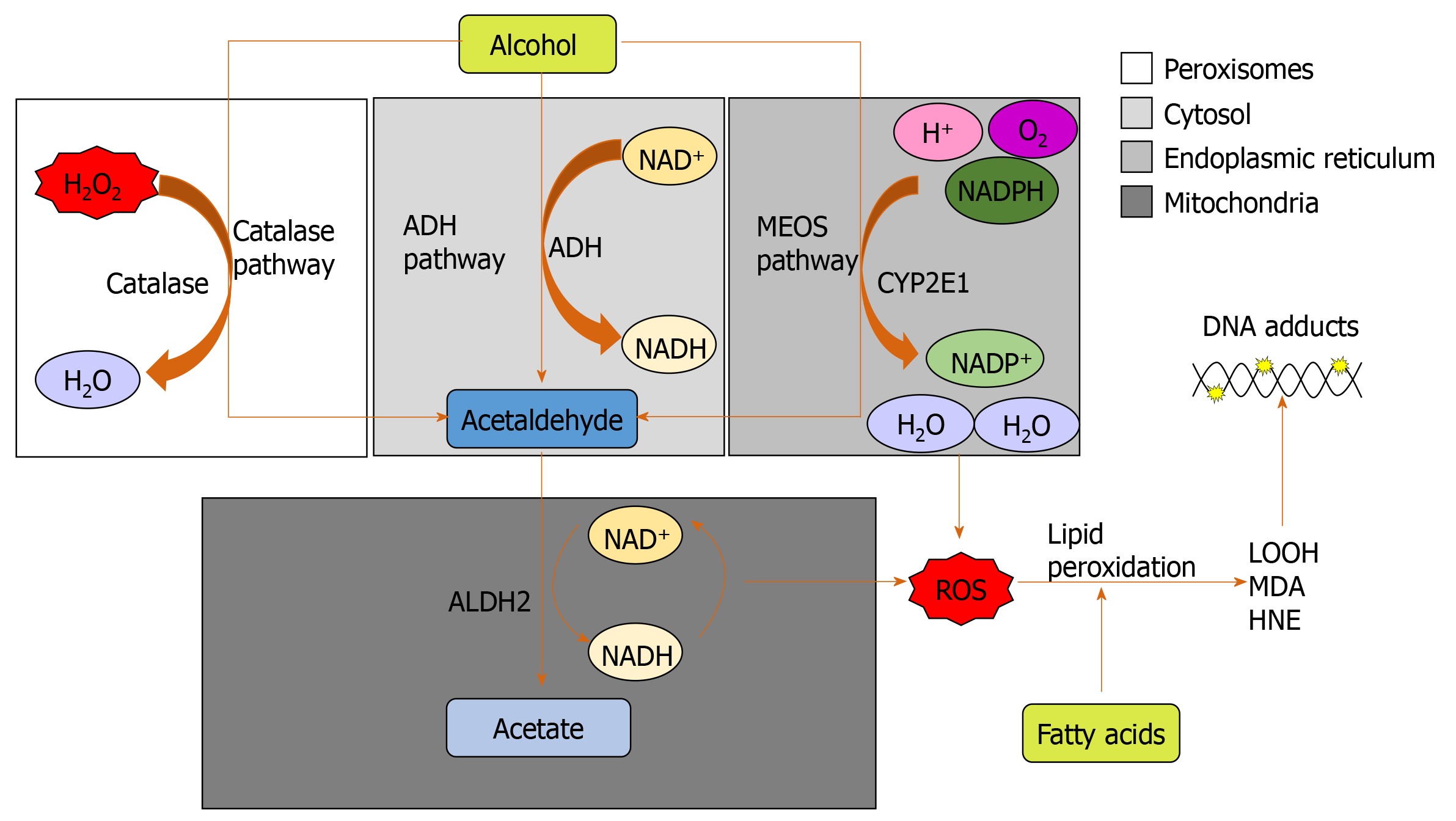
Figure 1 The three major pathways of alcohol metabolism.
The primary pathway is initiated by alcohol dehydrogenase, a NAD+ requiring enzyme expressed at high levels in hepatocytes, which oxidizes ethanol to acetaldehyde. The second major pathway, the microsomal ethanol oxidizing system pathway, involves the NADPH-requiring enzyme cytochrome P450 enzyme 2E1, which is induced by chronic alcohol exposure. The third pathway for ethanol metabolism is carried out by catalase, a peroxisomal enzyme. ADH: Alcohol dehydrogenase; ALDH2: Aldehyde dehydrogenase; CYP2E1: Cytochrome P450 enzyme 2E1; HNE: 4-hydroxy-2-nonenal; LOOH: Lipid hydroperoxides; MDA: Malondialdehyde; MEOS: Microsomal ethanol oxidizing system; ROS: Reactive oxygen species.
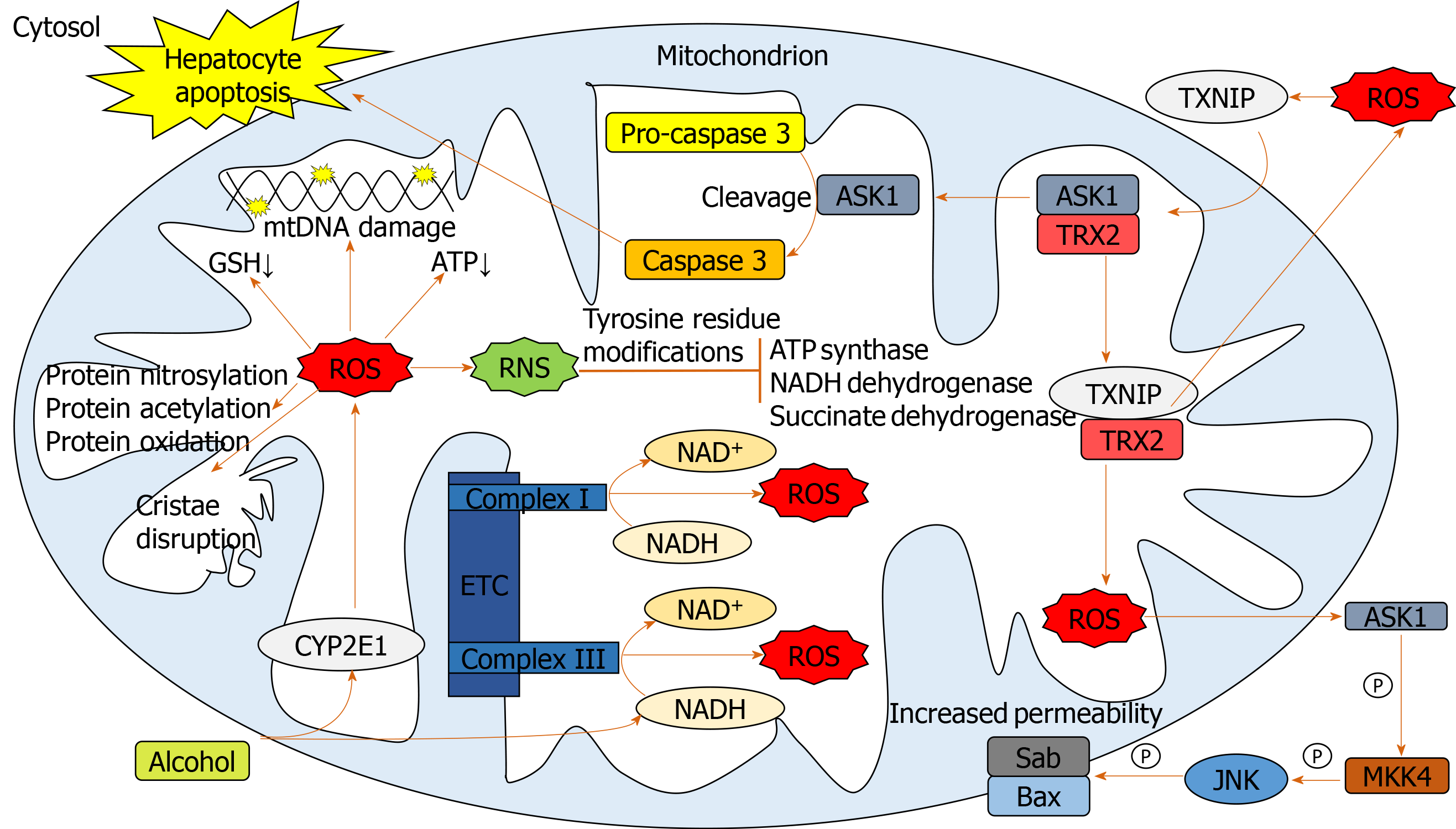
Figure 2 Pathways involved in mediating mitochondrial oxidative stress.
Alcohol elevates mitochondrial cytochrome p450 2E1 and NADH levels facilitating reactive oxygen species (ROS) upregulation. Elevated ROS damages mitochondrial DNA, proteins and cristae and causes a reduction in mitochondrial ATP and glutathione. ROS-activated thioredoxin-interacting protein translocates to mitochondria binding thioredoxin 2, indirectly producing further ROS through inhibiting its antioxidant activity. Apoptosis signal-regulating kinase 1 liberated from thioredoxin 2, facilitates cleavage of pro-caspase 3 to caspase 3 leading to hepatocellular apoptosis. Mitochondrial ROS activates cytosolic apoptosis signal-regulating kinase 1 leading to downstream opening of the mitochondrial transition pore through mitogen-activated protein kinase kinase 4 and c-Jun N-terminal kinase activation. ROS form reactive nitrogen species which inhibit mitochondrial enzymes. ASK1: Apoptosis signal-regulating kinase 1; BAX: Bcl-2-associated X protein; CYP2E1: Cytochrome p450 2E1; ETC: Electron transport chain; GSH: Glutathione; JNK: C-Jun N-terminal kinase; MKK4: Mitogen-activated protein kinase kinase 4; mtDNA: Mitochondrial DNA; ROS: Reactive oxygen species; RNS: Reactive nitrogen species; SAB: SH3 domain-binding protein that preferentially associates with Btk; TRX2: Thioredoxin 2; TXNIP: Thioredoxin-interacting protein.
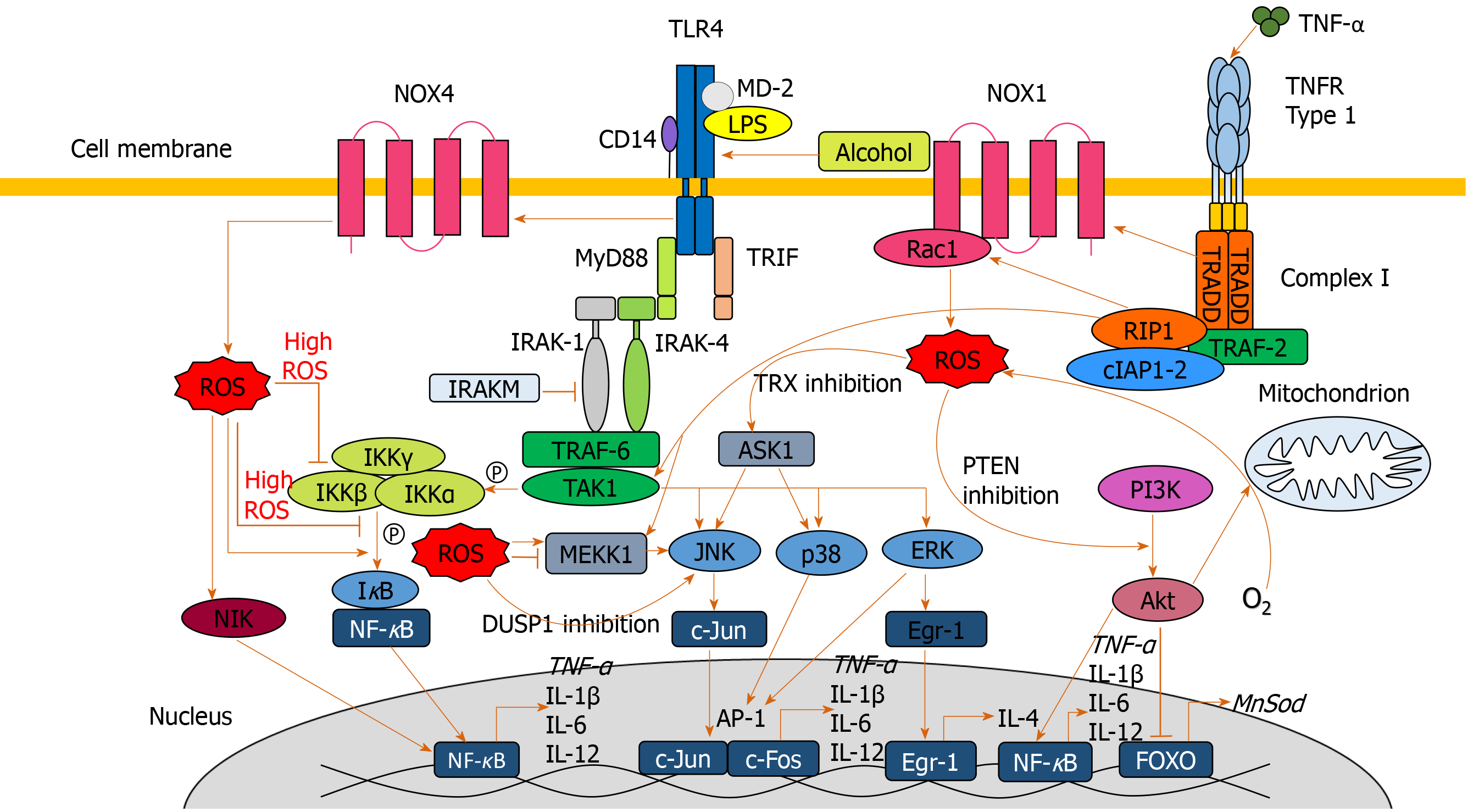
Figure 3 Signaling pathways involved in exacerbating oxidative damage and liver injury.
Lipopolysaccharide, alcohol and extracellular reactive oxygen species (ROS) are all capable of activating toll-like receptor 4 leading to myeloid differentiation primary response 88 (MyD88) activation. MyD88 association with interleukin-1 receptor-associated kinase 1-4 results in activation of the tumor necrosis factor receptor-associated factor 6/transforming growth factor beta-activated kinase 1 complex, which activates MAPKs c-Jun N-terminal kinase, p38 and extracellular signal-regulated protein kinase, facilitating transcription factors activator protein 1 and early growth response protein 1 to translocate to the nucleus and upregulate pro-inflammatory mediators. Tumor necrosis factor receptor-associated factor 6/transforming growth factor beta-activated kinase 1-mediated phosphorylation of the IKKα-β-γ complex leads to IκB phosphorylation and nuclear factor κB (NF-κB) translocation to the nucleus to upregulate pro-inflammatory cytokines. MyD88 signaling also activates NADPH oxidase 4 to produces ROS. ROS are also produced by the NADPH oxidase 1/ras-related C3 botulinum toxin substrate 1 complex which is activated upstream by tumour necrosis factor alpha interacting with tumour necrosis factor alpha receptor type 1, at the cell surface, which activates complex I. ROS upregulate NF-κB translocation to the nucleus through IκB phosphorylation, nuclear factor κB inducing kinase activation and indirect protein kinase B activation. At high concentrations, ROS inhibit NF-κB activation through inhibition of IκB phosphorylation and S-glutathionylation of IKKβ. ROS inhibit dual specificity protein phosphatase 1 and thioredoxin to further upregulate the c-Jun N-terminal kinase pathway. ROS inactivation of phosphatase and tensin homolog facilitates phosphoinositide 3-kinase to produce protein kinase B, which elevates ROS levels via increased oxygen consumption, and inactivates forkhead box protein O and downstream antioxidant expression. AKT: Protein kinase B; AP-1: Activator protein 1; ASK1: Apoptosis signal-regulating kinase 1; DUSP1: Dual specificity protein phosphatase 1; Egr-1: Early growth response protein 1; ERK: Extracellular signal-regulated protein kinase; FOXO: Forkhead box protein O; IAP: Inhibitor of apoptosis; IFN: Interferon; IL: Interleukin; IRAK: Interleukin-1 receptor-associated kinase 1; JNK: C-Jun N-terminal kinase; LPS: Lipopolysaccharide; MEKK1: Mitogen-activated protein kinase kinase kinase 1; MyD88: Myeloid differentiation primary response 88; NF-κB: Nuclear factor κB; NIK: Nuclear factor κB inducing kinase; NOX: NADPH oxidase; MnSOD: Manganese-dependent superoxide dismutase; PI3K: Phosphoinositide 3-kinase; PTEN: Phosphatase and tensin homolog; Rac1: Ras-related C3 botulinum toxin substrate 1; ROS: Reactive oxygen species; TAK1: Transforming growth factor beta-activated kinase 1; TLR4: Toll-like receptor 4; TNF-α: Tumour necrosis factor alpha; TNFR1: Tumour necrosis factor alpha receptor 1; TRADD: Tumour necrosis factor alpha receptor 1-associated death domain protein; TRAF: Tumor necrosis factor receptor-associated factor; TRIF: TIR-domain-containing adapter-inducing interferon-β; TRX: Thioredoxin; TXNIP: Thioredoxin-interacting protein.
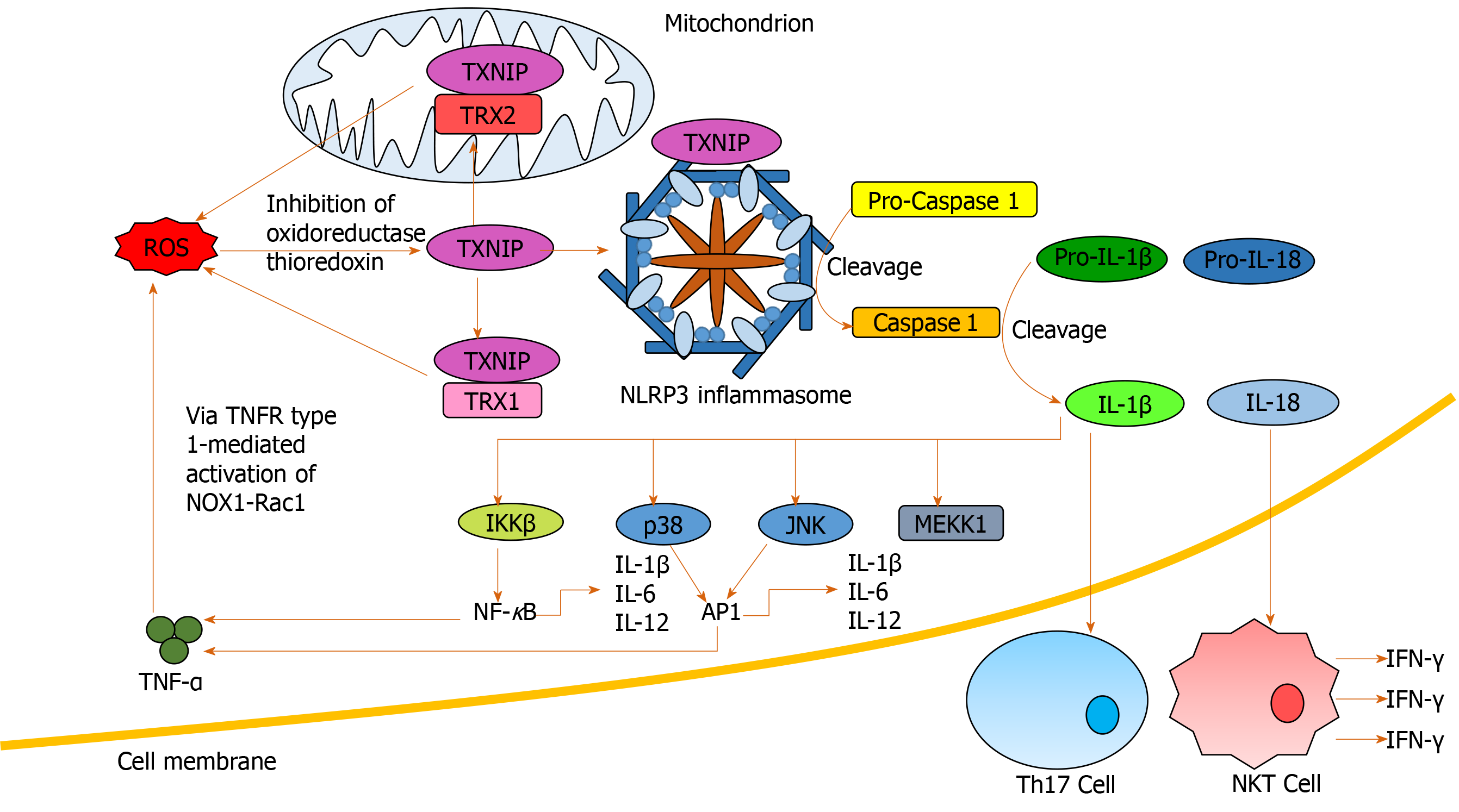
Figure 4 Nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 inflammasome activation and downstream signaling.
Reactive oxygen species (ROS) activate thioredoxin-interacting protein via inhibition of oxidoreductase thioredoxin. Thioredoxin-interacting protein both binds and activates nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 inflammasomes and interacts with thioredoxin 1 and 2 to indirectly promote further ROS generation through inhibiting their antioxidant activity. Activated nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 inflammasomes facilitate pro-caspase 1 cleavage to caspase 1, which facilitates pro- interleukin (IL)-1β and pro-IL-18 cleavage to IL-1β and IL-18 respectively. IL-18 induces interferon-γ production by natural killer T-cells. IL-1β induces generation of T-helper 17 cells in addition to nuclear factor κB and activator protein 1 activation through IKKβ, p38, c-Jun N-terminal kinase and mitogen-activated protein kinase kinase kinase 1 stimulation. Activating nuclear factor κB and activator protein 1 results in pro-inflammatory cytokine release, indirectly inducing further ROS accumulation. AP-1: Activator protein 1; IFN: Interferon; IL: Interleukin; JNK: C-Jun N-terminal kinase; MEKK1: Mitogen-activated protein kinase kinase kinase 1; NF-κB: Nuclear factor κB; NKT: Natural killer T-cell; NLRP3: Nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3; NOX: NADPH oxidase; Rac1: Ras-related C3 botulinum toxin substrate 1; ROS: Reactive oxygen species; Th17: T-helper 17 cells; TNF-α: Tumour necrosis factor alpha; TNFR1: Tumour necrosis factor alpha receptor 1; TRX: Thioredoxin; TXNIP: Thioredoxin-interacting protein.
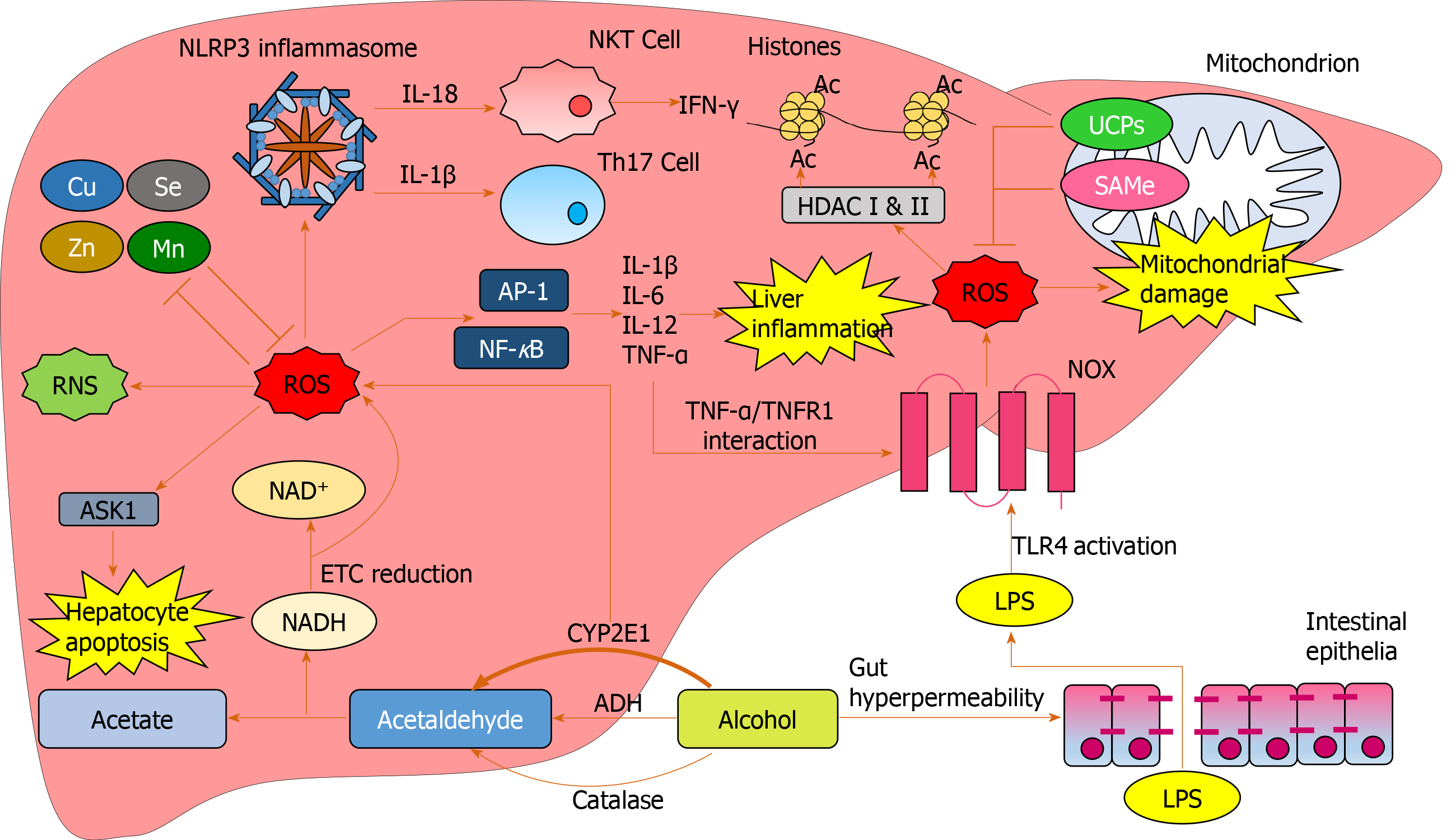
Figure 5 Reactive oxygen species-mediated oxidative damage in the liver.
Increased cytochrome p450 2E1-mediated alcohol breakdown and electron transport chain reduction results in overproduction of reactive oxygen species (ROS). Excess alcohol causes gut hyperpermeability resulting in tight junction disruption and an excess of lipopolysaccharide translocation from the gut to the liver. Lipopolysaccharide activates NADPH oxidase via toll-like receptor 4 activation resulting in further ROS production. Excess ROS produce RNS and reduce antioxidant cofactors such as Mn and Zn. ROS induce hepatocyte damage through activation of apoptosis signal-regulating kinase 1. Nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 inflammasomes are activated by ROS, inducing T-helper 17 generation and natural killer T cell-mediated interferon-γ production through interleukin expression. ROS upregulate transcription factors activator protein 1 and nuclear factor κB resulting in pro-inflammatory cytokine expression causing downstream liver inflammation. Tumour necrosis factor alpha further upregulates ROS through activating NADPH oxidase via tumour necrosis factor alpha receptor 1. ROS cause an array of functional and structural mitochondrial damage, which is initially impeded by uncoupling proteins and SAMe expression. ROS mediates epigenetic alterations through interacting with HDACs which mediate histone acetylation. Ac: Acetylation; ADH: Alcohol dehydrogenase; AP-1: Activator protein 1; ASK1: Apoptosis signal-regulating kinase 1; Cu: Copper; CYP2E1: Cytochrome p450 2E1; ETC: Electron transport chain; HDAC: Histone deacetylases; IFN: Interferon; IL: Interleukin; LPS: Lipopolysaccharide; Mn: Manganese; NF-κB: Nuclear factor κB; NKT: Natural killer T-cell; NOX: NADPH oxidase; ROS: Reactive oxygen species; SAMe: S-adenosylmethionine; Se: Selenium; Th17: T-helper 17 cells; TLR4: Toll-like receptor 4; TNF-α: Tumour necrosis factor alpha; TNFR1: Tumour necrosis factor alpha receptor 1; UCP: Uncoupling protein; Zn: Zinc.
- Citation: Tan HK, Yates E, Lilly K, Dhanda AD. Oxidative stress in alcohol-related liver disease. World J Hepatol 2020; 12(7): 332-349
- URL: https://www.wjgnet.com/1948-5182/full/v12/i7/332.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i7.332