Copyright
©The Author(s) 2020.
World J Hepatol. Nov 27, 2020; 12(11): 931-948
Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.931
Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.931
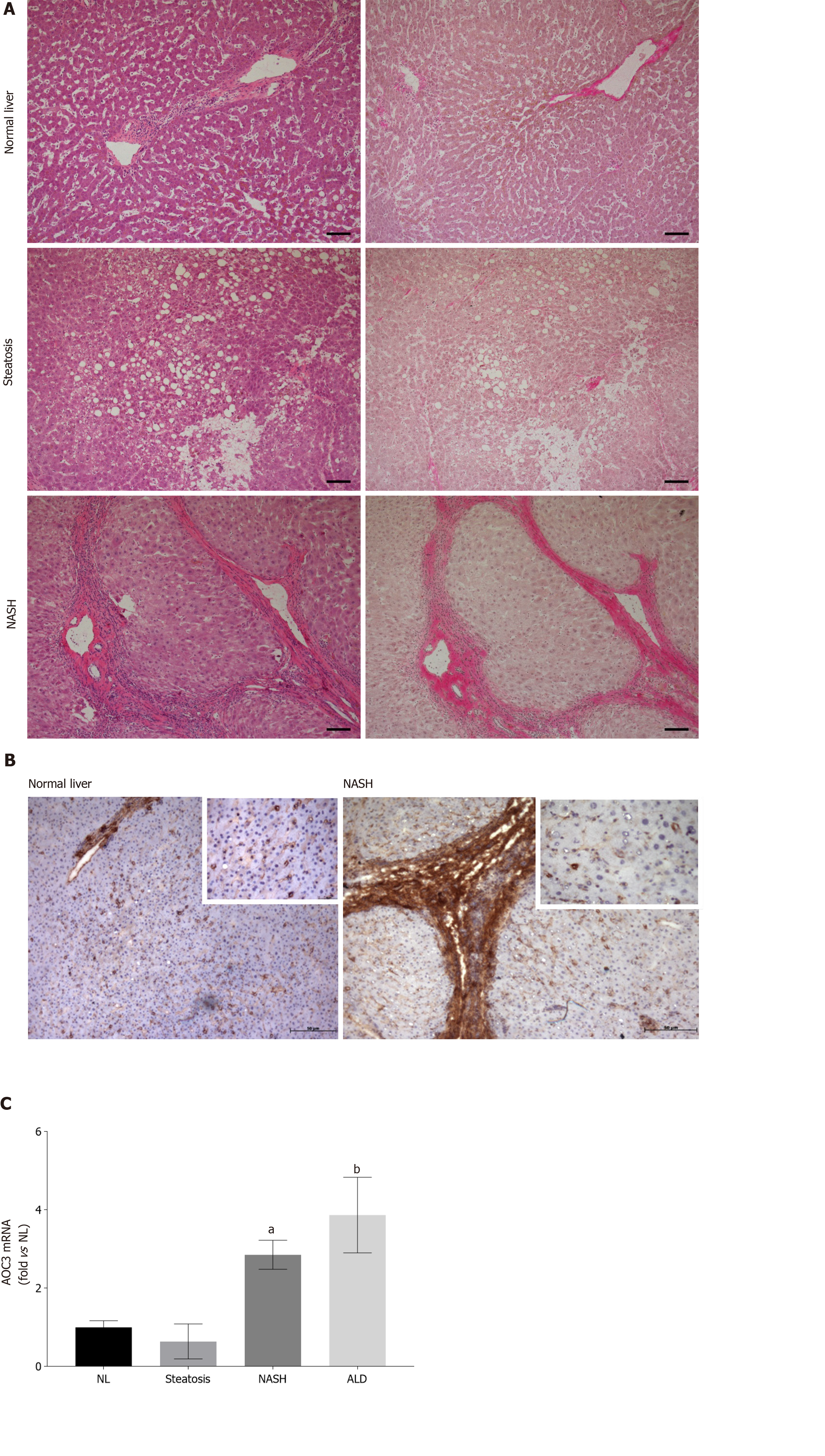
Figure 1 Hepatocellular expression of vascular adhesion protein-1 increases in nonalcoholic steatohepatitis.
A: Representative brightfield images of sections from indicated disease types stained with Hematoxylin and Eosin (left panels) and Van Geison’s Stain (right panels). Original magnification 10 ×, images representative of multiple fields of view from n = 3 Livers of each disease type. Scale bar is 100 µmol/L; B Immunohistochemical staining for vascular adhesion protein-1 (VAP-1) in representative acetone fixed frozen sections from normal and nonalcoholic steatohepatitis (NASH) livers. Isotype matched control antibody was negative (not shown). Fields were captured at 10 × original magnification with inset pictures captured at 40 × original magnification; C: Analysis of VAP-1 (AOC3) expression by quantitative qPCR analysis. mRNA expression of AOC3 in whole liver RNA from normal, steatotic, NASH, and alcohol-related cirrhosis (ALD) livers using fluidigm qPCR array®, run on triplicate arrays. Results are expressed as the mean fold change in gene expression normalized to pooled endogenous controls β-actin and GAPDH relative to normal livers defined as 1 ± SEM with means from five normal l, four steatotic, three NASH, and four ALD livers. aP < 0.05 or bP < 0.01 using a one way ANOVA with Bonferroni correction. NASH: Nonalcoholic steatohepatitis; ALD: Alcohol-related cirrhosis.
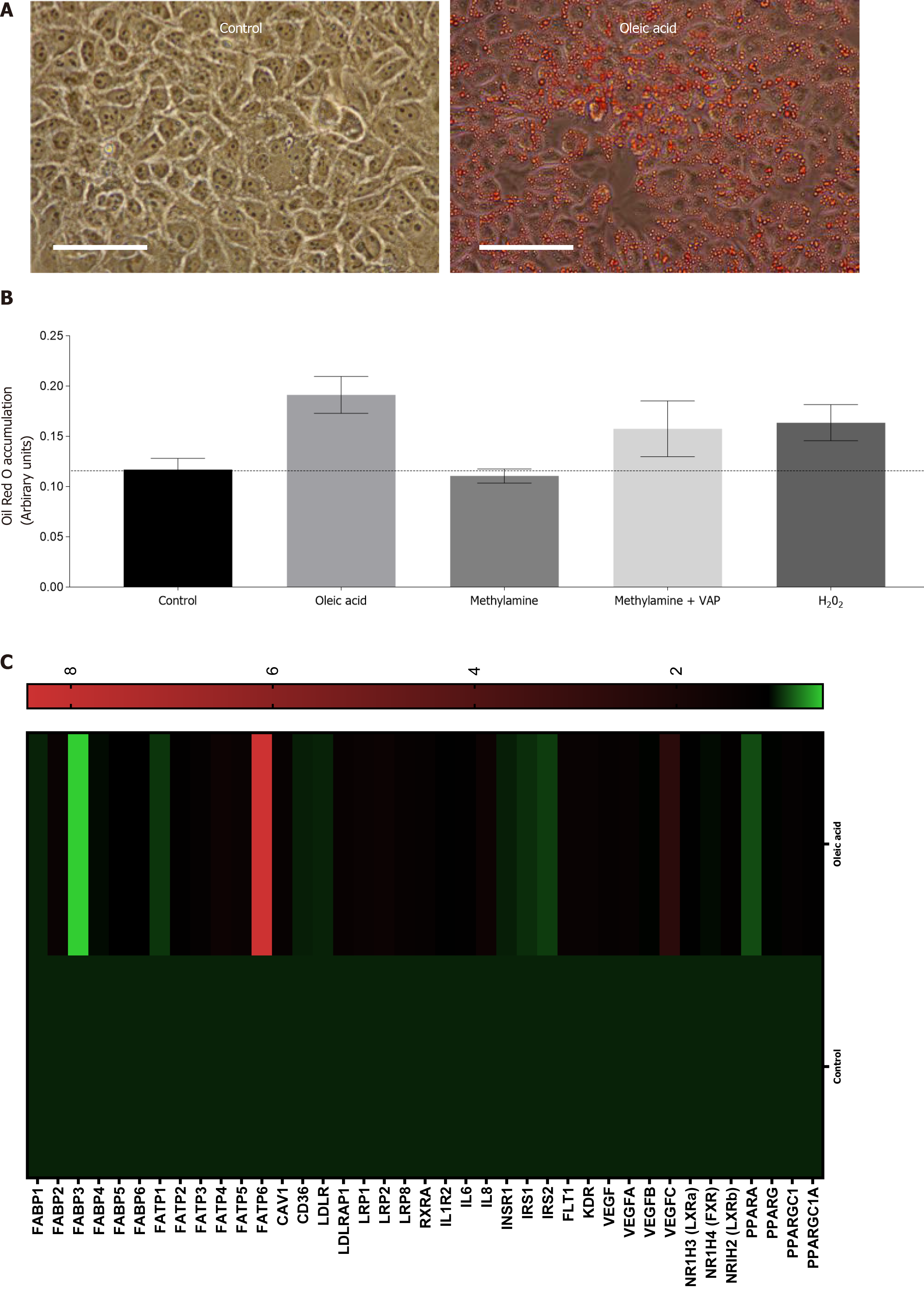
Figure 2 Exposure of Huh7.
5 cells to products of vascular adhesion protein-1 enzyme activity leads to lipid accumulation and gene expression changes. A: Representative phase contrast images of confluent control Huh7.5 (left) or cells pretreated with 250 μm Oleic Acid for 6 h. All wells were fixed and stained with Oil Red O, and images were captured at 40 × original magnification (representative of n = 3 samples per condition). Bar is 50 mm; B: Quantification of oleic acid accumulation after vascular adhesion protein-1 (VAP-1) stimulation. Huh7.5 were pretreated with either methylamine (200 μm) alone, or in combination with recombinant VAP-1 (500 ng/mL) or H2O2 (10 μmol/L), for approximately 18 h. This was followed by incubation for 6 h with 250 μm OA. Cells were fixed and stained with Oil Red O and solubilized. Signal is expressed in arbitrary units. Data are mean ± SEM of triplicate experiments; C: Analysis of mRNA expression in HuH7.5 cells after exposure to oleic acid by quantitative qPCR analysis. mRNA expression for indicated genes was assessed using fluidigm qPCR array® according to manufacturer’s instructions. Data is expressed as fold changes in relative gene expression compared to pooled housekeeping genes in control (untreated cells). Data are representative of triplicate conditions run on triplicate gene array plates.
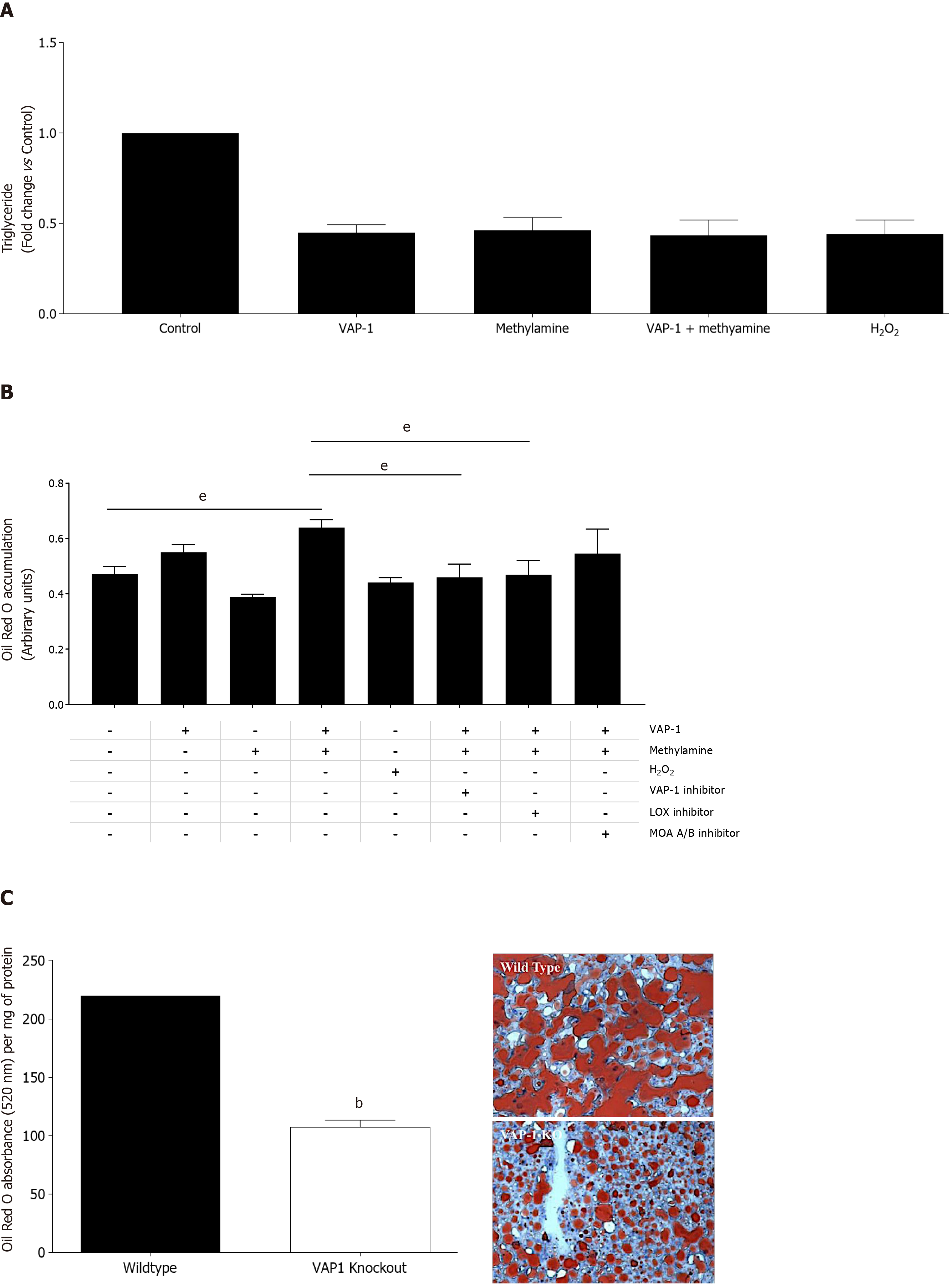
Figure 3 Activation of vascular adhesion protein-1 enzyme activity results in reduced triglyceride export and increased steatosis in human liver tissue and VAP-1/AOC3 knockout protects against high fat diet induced steatosis in mice.
A: PCLS were pretreated with either, methylamine 200 μm, vascular adhesion protein-1 (VAP-1) 500 ng, H2O2 10 μmol/L, or a combination of methylamine + VAP-1, benzylamine+VAP-1 for approximately 18 h and then 6 h with 250 μm fatty acid. Supernatants were collected after treatments and triglyceride secretion was quantified using a commercial assay (Cayman Chemical Company) according to manufacturer’s instructions. Data are triplicate samples from n = 2 normal livers ± SEM. P < 0.01 for all using a one way ANOVA; B: Lipid uptake in PCLS from normal liver tissue pretreated with either methylamine 200 μm, and rVAP1 500 ng/mL alone or in combination, or, H2O2 10 μmol/L. Some slices were exposed to the combination of methylamine and VAP-1 plus selective enzyme inhibitors: Bromoethylamine (VAP-1 inhibitor 400 μm), β-aminopropionitrile (lysyl oxidase inhibitor, BAPN 250 μm) or the Monoamine oxidase A and B inhibitors Clorgylline and Pargylline (both at 200 μm). After approximately 18 h incubation, slices were exposed to 250 μm oleic acid for 6 h. PCLS were fixed and stained with Oil RedO, which was solubilized and signal normalized to per 500 mg of tissue. Data are mean of triplicate samples from n = 2 normal livers ± SEM. Significance expressed as eP < 0.001 in one way ANOVA with Tukeys correction for multiple comparisons; C: Left - Accumulation of lipid in WT and VAP-1 KO mice fed on a high fat diet for 12 wk. 7um cryosections from WT and VAP-1 KO mouse livers were stained with ORO, which was then solubilized and signal expressed relative to protein concentration for each group of mice, Data are mean ± SEM of three mice per group. Significance expressed as bP < 0.01 one way ANOVA. Right – representative brightfield microscopy images of Oil red O stained cryosections from WT and VAP-1 KO mice.
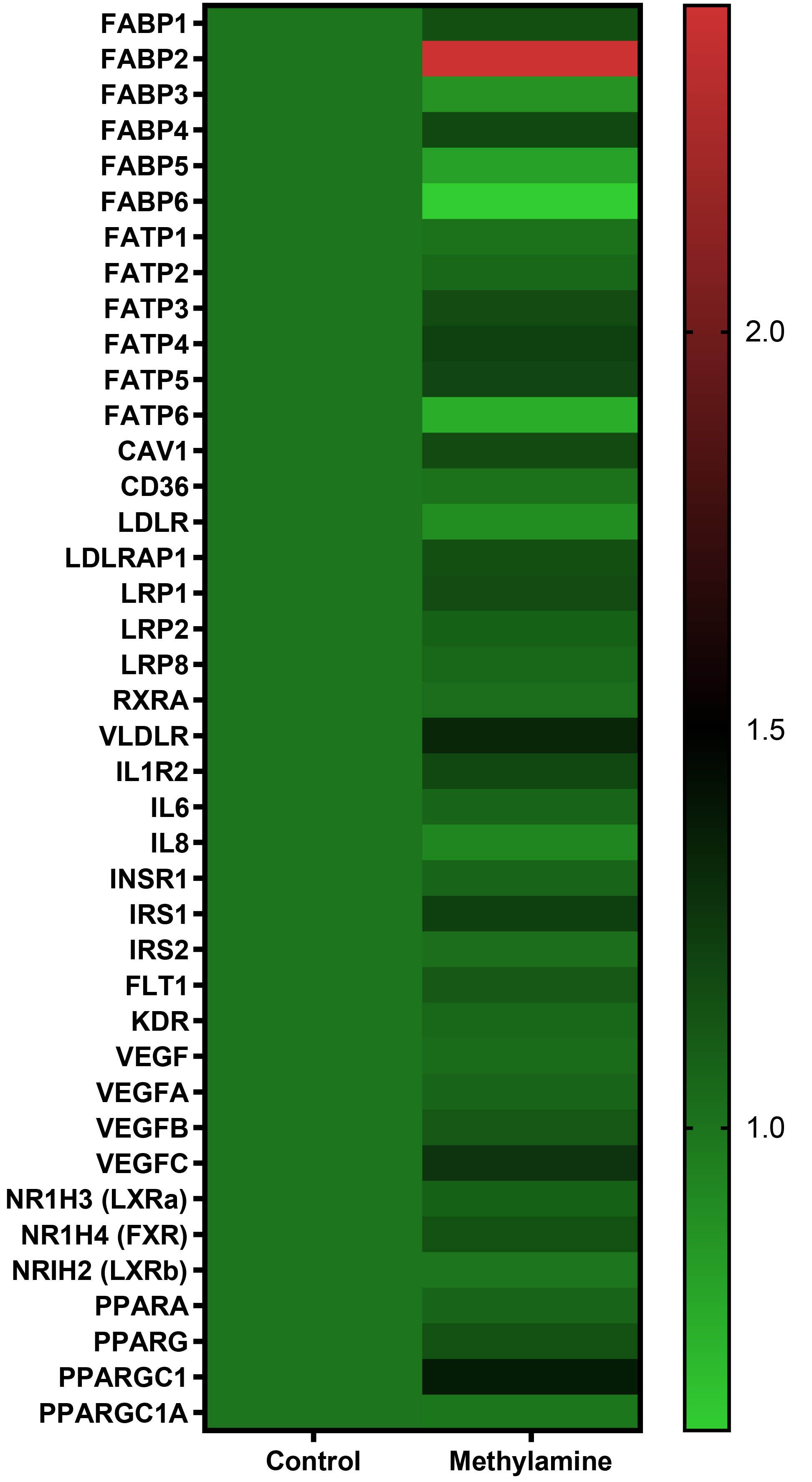
Figure 4 Exposure of precision-cut liver slices from human donor liver tissue cells to substrate for vascular adhesion protein-1 enzyme activity leads to gene expression changes.
Precision-cut liver slices were treated with methylamine 200 μm for approximately 4.5 h. RNA was extracted and mRNA expression was carried out using a fluidigm qPCR array® run on triplicate arrays. Results are expressed as the mean fold change in gene expression normalized to pooled endogenous controls β-actin and GAPDH relative to untreated control livers. Data are indicative of triplicate arrays prepared from 2 donor livers.
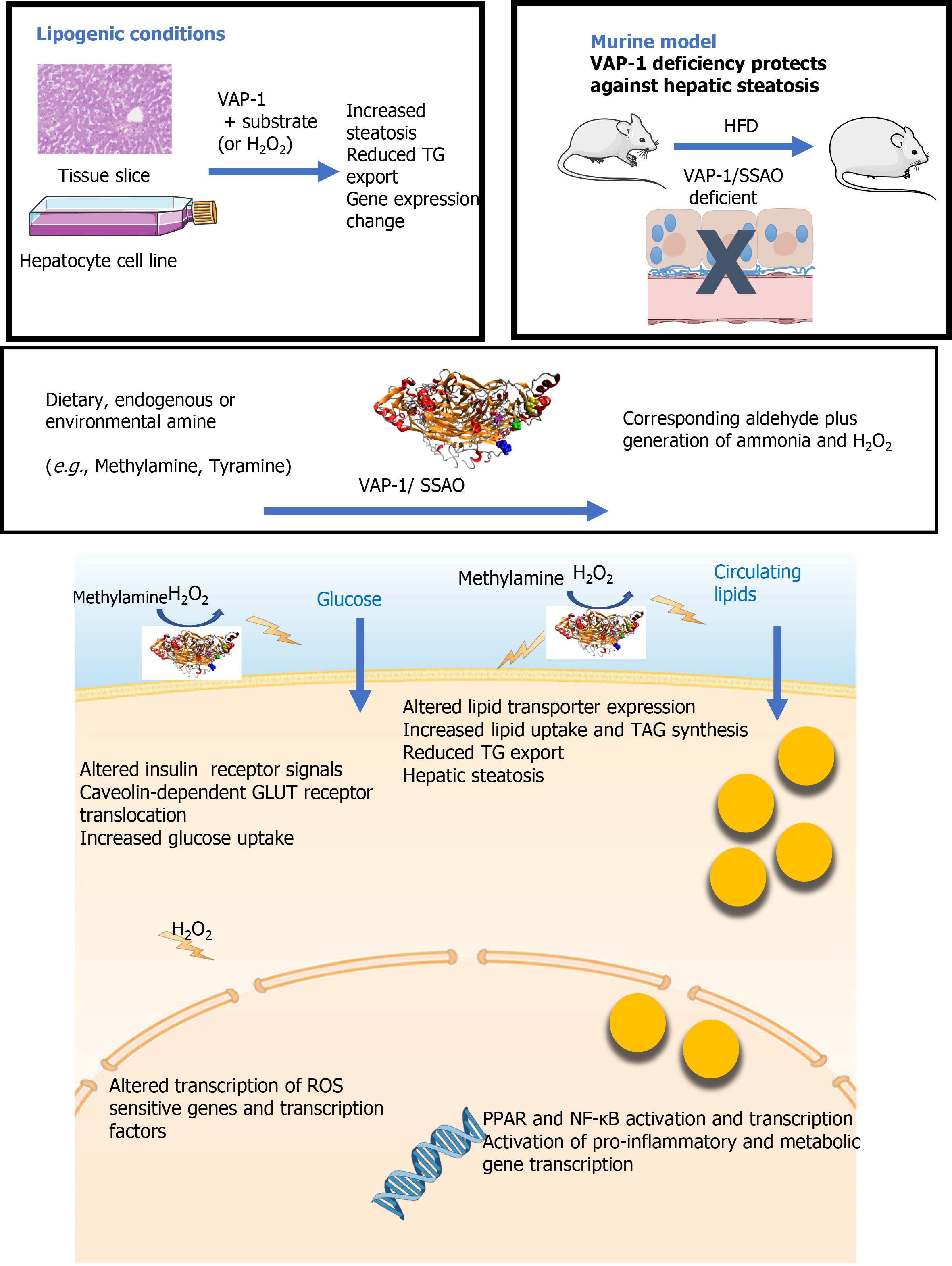
Figure 5 Graphical summary the impact of vascular adhesion protein-1 on the hepatic pathogenesis of non-alcoholic fatty liver disease.
Exposure of human liver tissue or hepatocytes in culture to vascular adhesion protein-1 (VAP-1) in the presence of endogenous or exogenous (methylamine) substrate led to a reduction in TG export and increased steatosis. In tissue this was accompanied by changes in metabolic gene expression. VAP-1 deficient mice are protected against hepatic steatosis when fed a high fat diet. These findings can be explained by the enzymatic capacity of VAP-1 to reduce amine substrates to the corresponding aldehyde accompanied by generation of potent signaling molecules such as hydrogen peroxide. Addition of hydrogen peroxide to our culture systems recapitulated the effects of VAP-1 activation. Our previous studies suggest that increased inflammation, steatosis and fibrosis in the context of non-alcoholic fatty liver disease in part relate to the ability of VAP-1 to support leukocyte recruitment across endothelial cells, to prime hepatic glucose uptake and to activate hepatic stellate cells. We now show additional effects on transcription of key lipid transporter molecules and transcription factors. The upregulation of FABP4, FABP2, FATP3-5 and LRP1 along with the VLDLR which alter uptake and intracellular targeting of lipid molecules, Transport of fatty acids to the nucleus by receptors such as FABP2 will also activate nuclear receptors such as PPARs and NF-κB, hence influencing gene transcription. VAP-1: Vascular adhesion protein-1; HFD: High fat diet.
- Citation: Shepherd EL, Karim S, Newsome PN, Lalor PF. Inhibition of vascular adhesion protein-1 modifies hepatic steatosis in vitro and in vivo. World J Hepatol 2020; 12(11): 931-948
- URL: https://www.wjgnet.com/1948-5182/full/v12/i11/931.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i11.931