Copyright
©The Author(s) 2018.
World J Hepatol. Mar 27, 2018; 10(3): 352-370
Published online Mar 27, 2018. doi: 10.4254/wjh.v10.i3.352
Published online Mar 27, 2018. doi: 10.4254/wjh.v10.i3.352
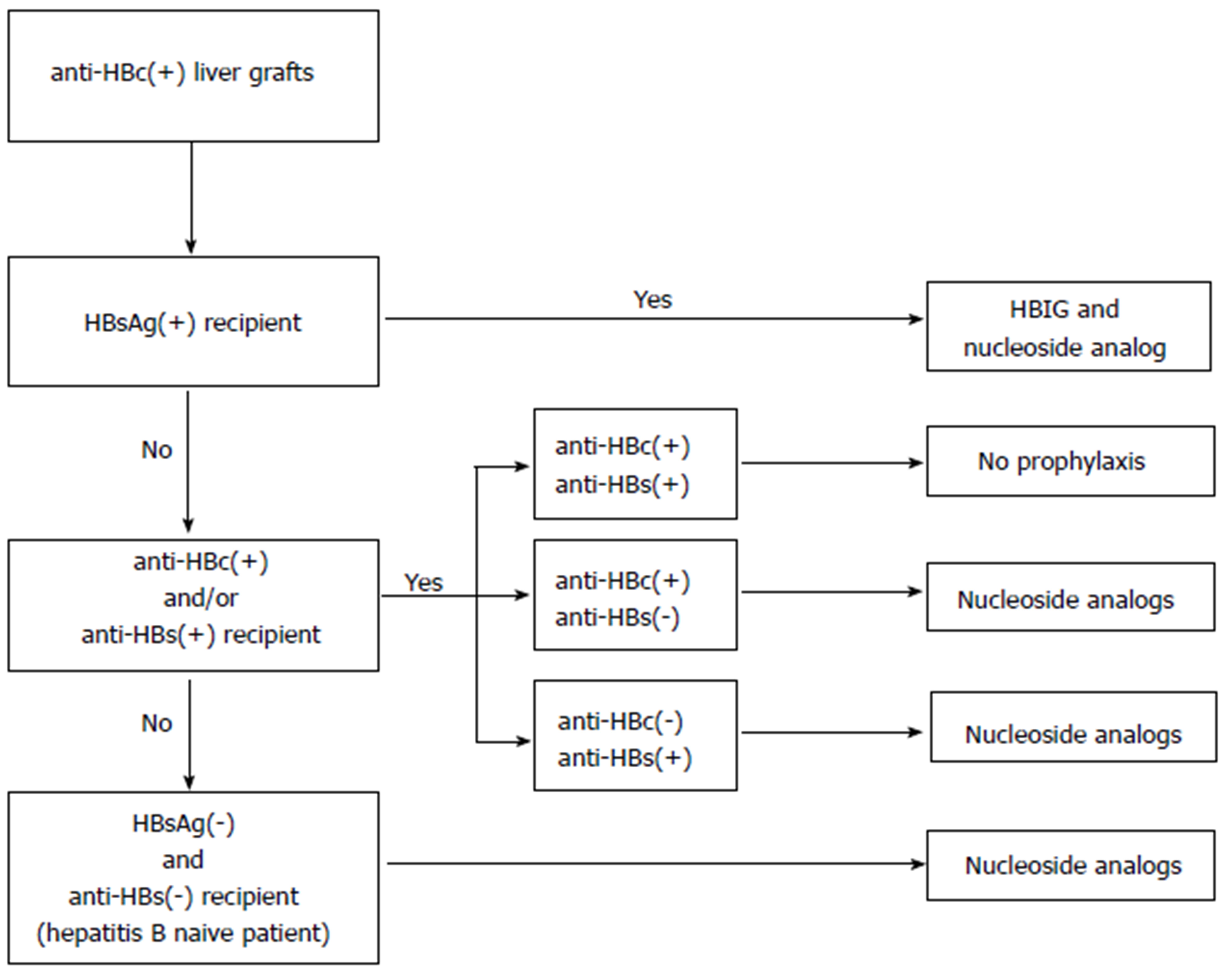
Figure 1 Stepwise approach of anti-hepatitis B core positive grafts allocated to recipients based on their hepatitis B serology.
In chronic hepatitis B patients with HBsAg positive and who receive Anti-HBc positive liver grafts should be treated with HBIG and nucleoside analogs. If the recipient is HBsAg negative and Anti-HBc positive and/or anti HBs positive, NA is used for prophylaxis based on anti HBc and anti HBs serologies. No prophylaxis is recommended for anti-HBc positive and anti-HBs positive liver in LT recipient without HBsAg positive serology. These patients should be followed with periodic HBV DNA level guided by ALT to monitor for any relapse. In Hepatitis B naïve patients, NA is recommended for prophylaxis. HBIG: Hepatitis B Immunoglobulin; HBsAg: Hepatitis B surface antigen; Anti-HBs: Hepatitis B surface antibody; Anti-HBc: Hepatitis B core antibody.
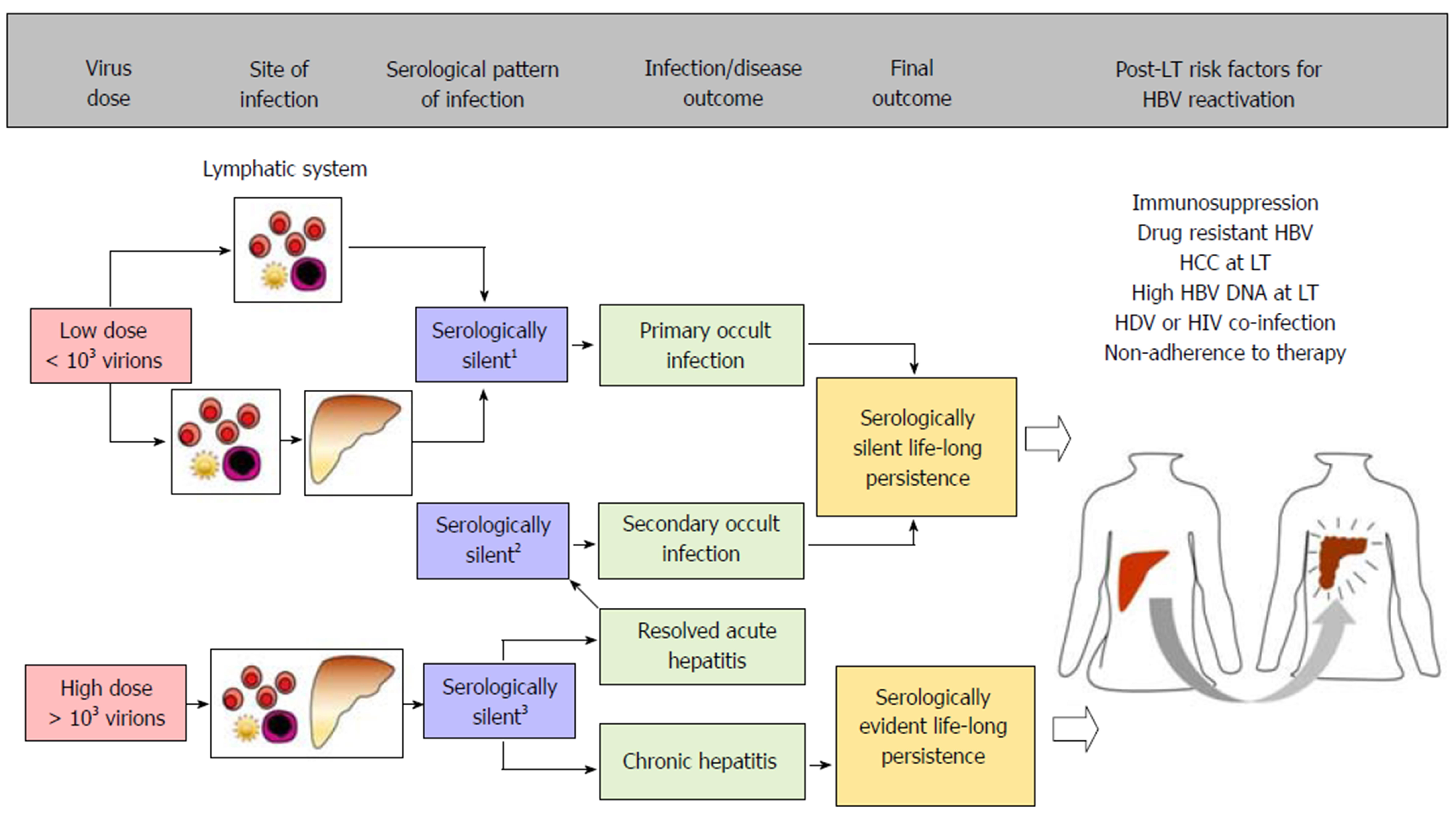
Figure 2 Generalized concept of overt and occult hepatitis B virus infections based on the data from the woodchuck model of hepatitis B, their long-term outcomes, and associated risk factors for hepatitis B virus reactivation following liver transplant.
Based on experimental infection in the woodchuck model (Mulrooney-Cousins PM, Michalak TI, 2015[92]). 1Serologically silent infection: HBsAg, anti-HBc and anti-HBs negative; HBV DNA positive; 2Serologically silent infection: HBsAg negative, anti-HBc positive, anti-HBs positive or negative; HBV DNA positive; 3Serologically evident infection: HBsAg and anti-HBc positive, anti-HBs negative. HBV DNA positive. SOI: Secondary occult infection; POI: Primary occult infection; LT: Liver transplant; HCC: Hepatocellular carcinoma; HBV: Hepatitis B virus; HDV: Hepatitis D virus; HBsAg: HBV surface antigen; anti-HBc: Antibodies to HBV core antigen; anti-HBs: Antibodies to HBV surface antigen.
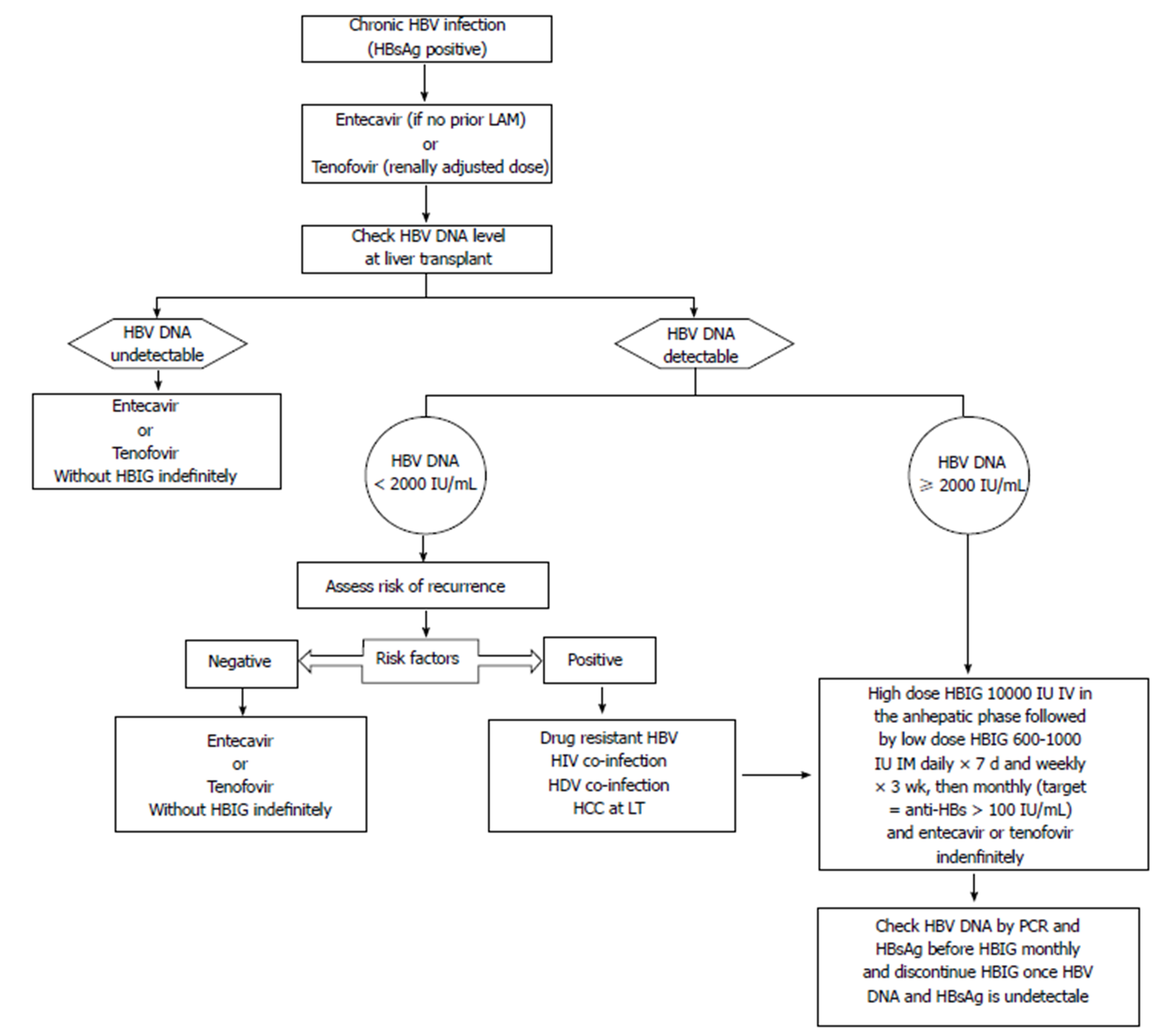
Figure 3 Proposed algorithm for hepatitis B prophylaxis in liver transplant patients.
In chronic hepatitis B patients Entecavir (if no prior Lamivudine therapy) or Tenofovir (adjusted to renal function) is recommended as the first line therapy. Based on HBV DNA level at the time of transplant and risk factors, HIBG should be initiated, if associated risk factors for HBV recurrence post LT. High risk patients include drug resistant HBV, HIV co-infection, HDV co-infection, HCC. This group of patients receive high dose IV HBIG 10000 IU given during the anhepatic phase followed by low dose HBIG to achieve target anti HBs > 100 IU/mL along with NAs. HBIG is discontinued once HBV DNA is undetectable and loss of HBsAg is achieved. HBIG: Hepatitis B immunoglobulin; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; HCC: Hepatocellular carcinoma; HIV: Human immunodeficiency virus; HDV: Hepatitis delta virus; LAM: Lamivudine; LT: Liver transplantation.
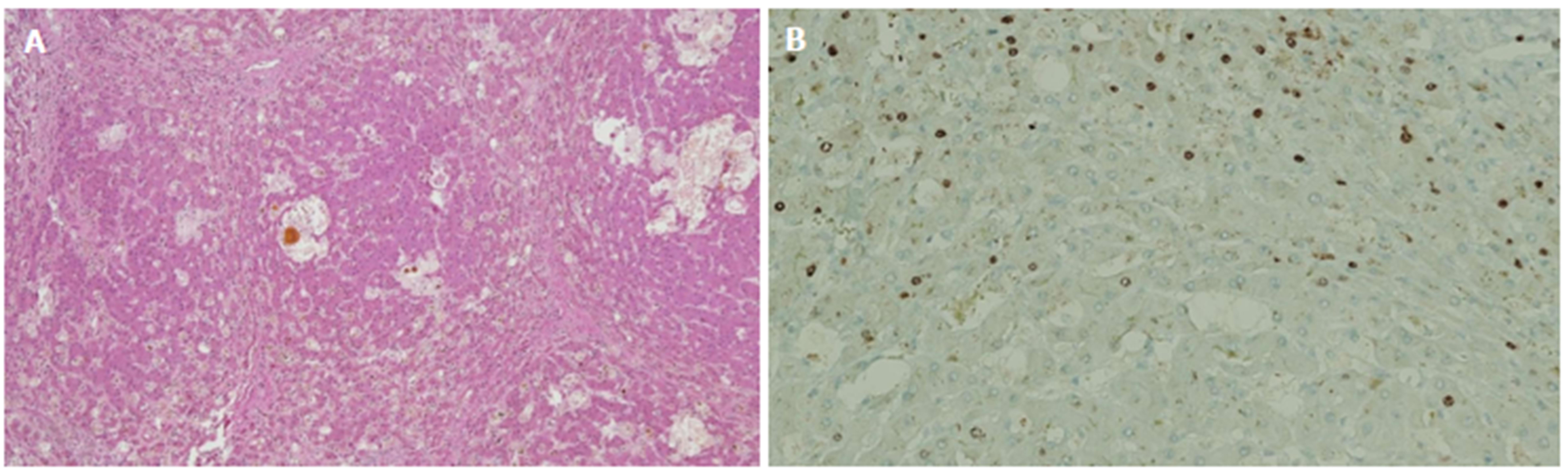
Figure 4 Immunostaining.
A: Recurrent hepatitis B virus infection leading to cirrhosis in a post-liver transplantation patient. Figure shows cirrhotic nodules with cholestasis but no appreciable inflammation; B: Immunostaining for hepatitis B core antigen shows strong nuclear accumulation of antigen in a small proportion of hepatocytes indicating active virus propagation.
- Citation: Chauhan R, Lingala S, Gadiparthi C, Lahiri N, Mohanty SR, Wu J, Michalak TI, Satapathy SK. Reactivation of hepatitis B after liver transplantation: Current knowledge, molecular mechanisms and implications in management. World J Hepatol 2018; 10(3): 352-370
- URL: https://www.wjgnet.com/1948-5182/full/v10/i3/352.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i3.352