Published online Nov 26, 2019. doi: 10.4252/wjsc.v11.i11.904
Peer-review started: March 22, 2019
First decision: August 1, 2019
Revised: August 10, 2019
Accepted: September 13, 2019
Article in press: September 13, 2019
Published online: November 26, 2019
Processing time: 229 Days and 1 Hours
Both parasitology and stem cell research are important disciplines in their own right. Parasites are a real threat to human health causing a broad spectrum of diseases and significant annual rates morbidity and mortality globally. Stem cell research, on the other hand, focuses on the potential for regenerative medicine for a range of diseases including cancer and regenerative therapies. Though these two topics might appear distant, there are some “unexpected encounters”. In this review, we summarise the various links between parasites and stem cells. First, we discuss how parasites’ own stem cells represent interesting models of regeneration that can be translated to human stem cell regeneration. Second, we explore the interactions between parasites and host stem cells during the course of infection. Third, we investigate from a clinical perspective, how stem cell regeneration can be exploited to help circumvent the damage induced by parasitic infection and its potential to serve as treatment options for parasitic diseases in the future. Finally, we discuss the importance of screening for pathogens during organ transplantation by presenting some clinical cases of parasitic infection following stem cell therapy.
Core tip: The aim of this review was to bring together two important research disciplines: parasitology and stem cell biology. Parasites are the caustic agents of numerous diseases that have a huge impact on human health. The regeneration properties of stem cells are remarkable and the exploitation of such biology for clinical applications is an exciting area of research that may provide future treatment options for a wide range of human diseases. These apparently independent fields of research have multiple areas of overlap which we cover highlighting that these interactions, particularly from a clinical perspective, should be more seriously considered and studied.
- Citation: Matthews H, Noulin F. Unexpected encounter of the parasitic kind. World J Stem Cells 2019; 11(11): 904-919
- URL: https://www.wjgnet.com/1948-0210/full/v11/i11/904.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i11.904
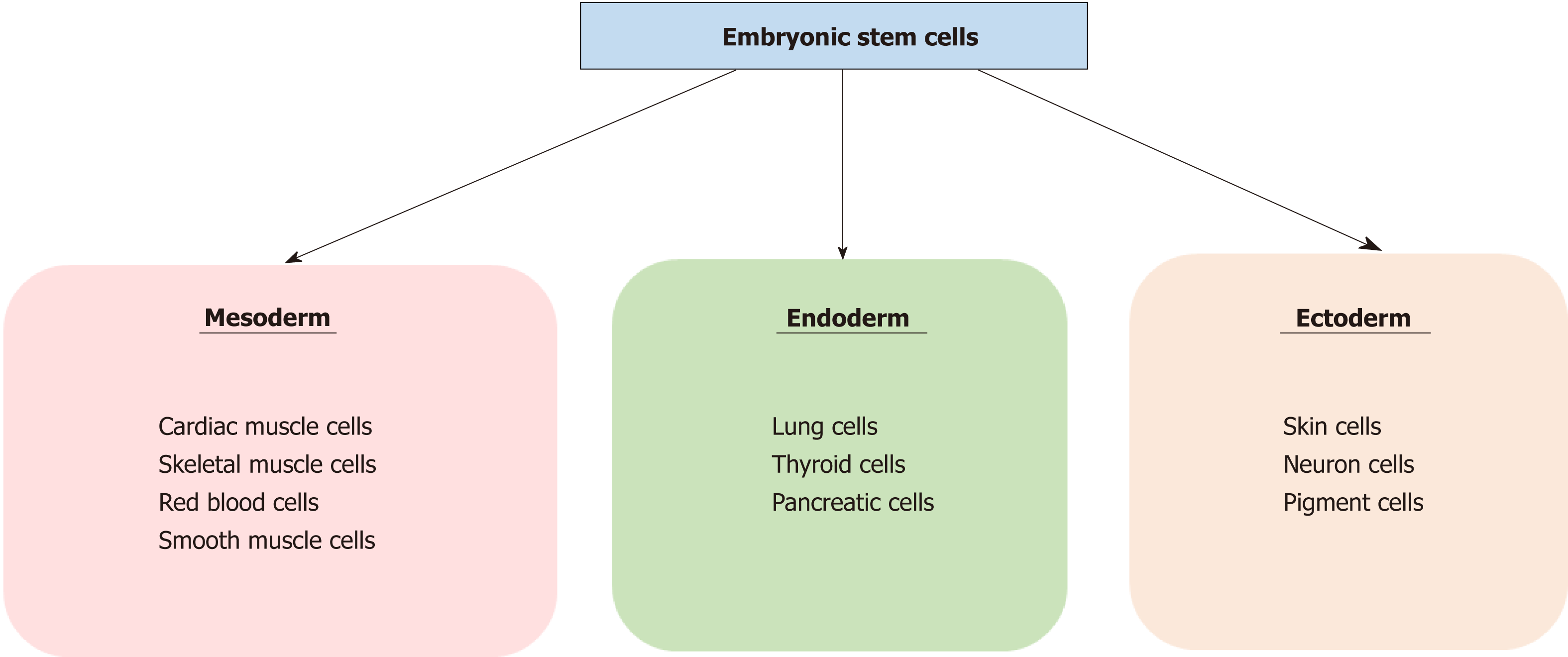
Stem cells are defined by their self-renewal properties, and their ability to differentiate into adult cells[1]. They can be classified into two groups according to their biological properties: Pluripotent and multipotent stem cells. Pluripotent stem cells are able to differentiate into any cell type, and are found in the blastocyst, and early embryonic developmental stage[2]. Pluripotent stem cells give rise to three germ layers (endo-derm, mesoderm and ectoderm) that ultimately produce different organs: cardiac, skeletal muscle or red blood cells for the mesoderm, pancreatic or lung cells for the endoderm, and neuron or skin cells for the ectoderm (Figure 1). Multipotent stem cells are more limited with regards to their differentiating capabilities. While they can differentiate into multiple cell types, they are already polarised down a specific route and can be found in most adult tissues where they replace aged or damaged cells throughout the lifespan of the multicellular organism[3].
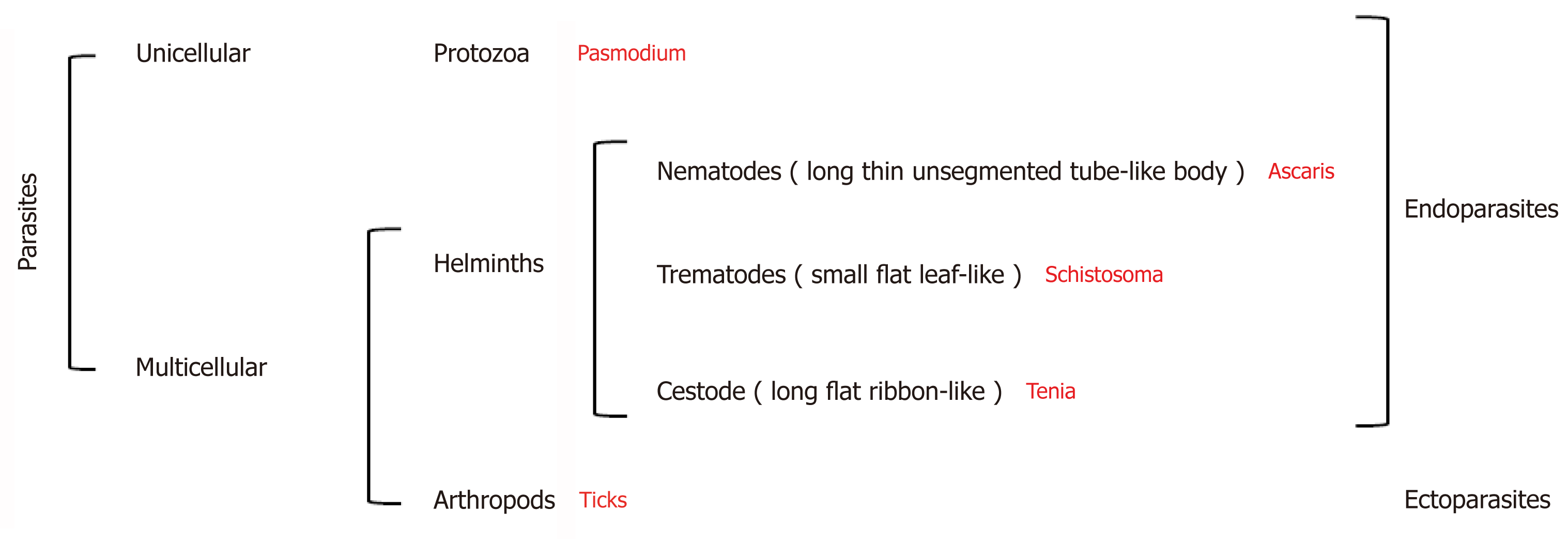
Parasites are organisms that survive by taking nutrients from another organism: their host[4]. They represent more than 50% of all animal species[5] and can be broadly divided into three groups: Protozoa, helminths, and ectoparasites (Figure 2). The diseases they cause inflict huge health and socio-economic burden on low income countries[6]. Indeed, parasitic infections were considered responsible for 7.2% of deaths globally in 2016[7].
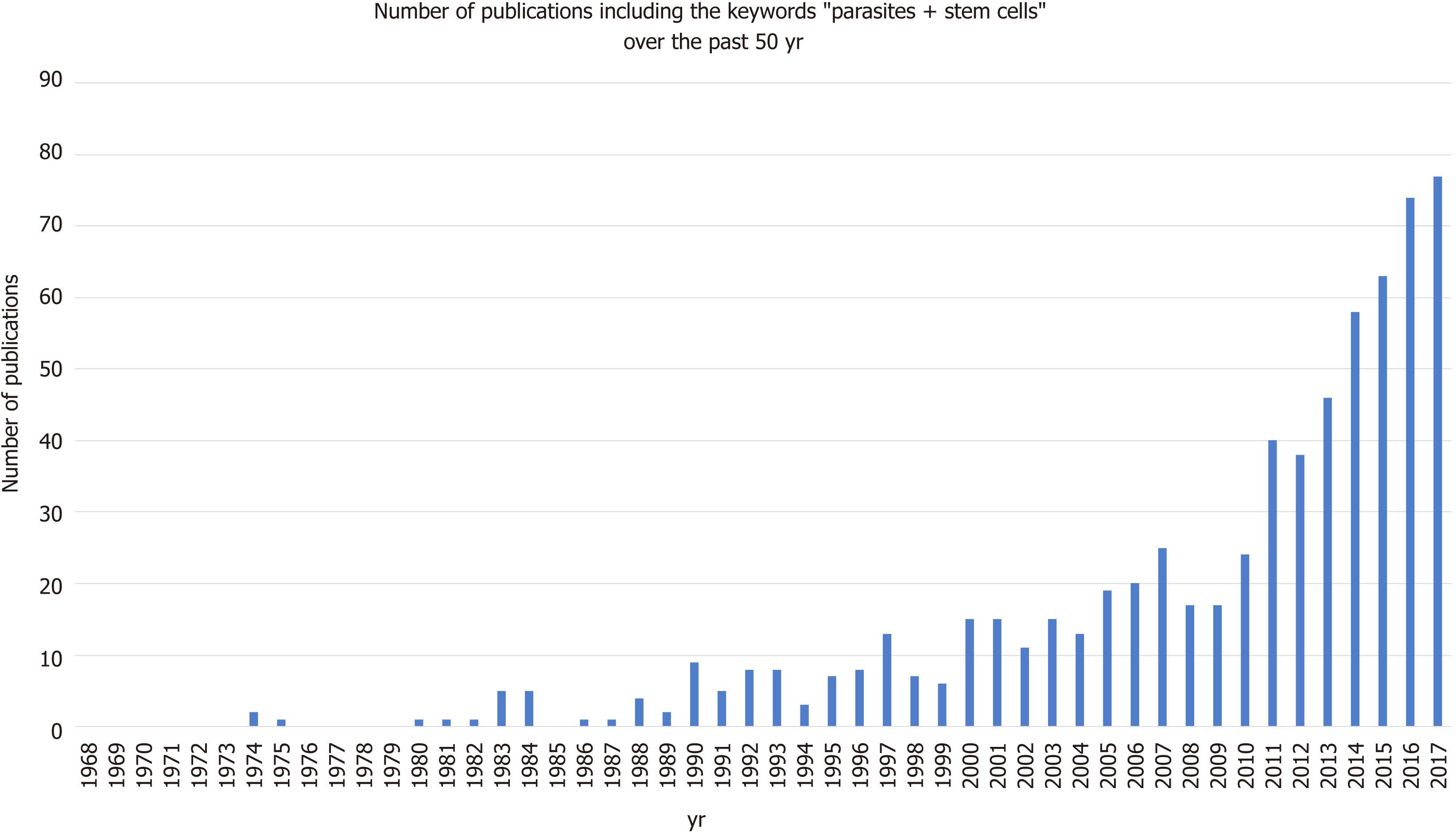
The relationship between parasites and stem cells is becoming increasingly studied (Figure 3). The purpose of this review is to further examine the relationship between stem cells and parasites, specifically looking at parasitic stem cells, how the parasites influence the host’s stem cells, and how these interactions may be exploited clinically.
Although unicellular protozoan parasites do not have stem cells, the stems cells of other multi-cellular parasites such as helminths have been studied. Interestingly, one of the earliest references to the term “stem cell” was in relation to the parasitic helminth Ascaris megalocephala in the late 19th century[8]. Thus, it is perhaps unsurprising that some parasite’s stem cells have been used to better understand the regeneration system.
The tapeworm Echinococcus is one such parasite. This organism presents primarily as a zoonosis but can infect humans through animal transmission[9]. While the infection can manifest in four distinct forms, only two are relevant to human health: cystic and alveolar. Cystic Echinococcus infection is caused by Echinococcus granulosus and is characterised by the development of hydatid cysts, typically in the liver and lungs. Alveolar Echinococcus infection is caused by E. multilocularis and is initially asymptomatic, but a primary tumour-like lesion develops in the liver. This form is fatal if untreated. The Echinococcus life cycle begins when the adult (located in the intestine of the definitive Canidae host) releases eggs that exit the host in the faeces. Once ingested by an intermediate host, i.e. sheep, the eggs hatch and release oncospheres that pass through the intestinal wall and migrate into different organs. There, the larval forms develop into cysts containing protoscolices: the form that is ingested by the definitive host that evolves into protoscolex. Following ingestion, the protoscolices attach to the intestinal mucosa where they develop into adults.
Recently, Koziol et al[10] proposed to identify germinal cells in E. granulosus. For this, they treated the parasites cells with 5-Ethynyl-2´-deoxyuridine (EDU), a synthetic nucleotide that is incorporated into newly synthesised DNA, to identify cells in proliferation. They noticed that the germinal cells were less proliferative during the protoscolex stage but were reactivated when ingestion by a host was mimicked, highlighting the ability of these cells to remain quiescent while not in optimal conditions. To better characterise this cell population, they proposed to identify potential specific marker genes using the whole mount in situ hybridisation (commonly known as WMISH) technique but unfortunately were unsuccessful in this attempt. Notably, germinative cells could not be fully eliminated after gamma radiation treatment and the parasite only showed a delayed growth defect. From all these observations, they concluded that some parasite cells are capable of self-renewal and differentiation into proliferative competent cells.
In further work focusing on mobile genetic elements, Koziol et al[11], identified a novel family of terminal-repeat retrotransposons in miniature (known as TRIMs) as potential germline cell markers. Using a computer modelling approach, they identified putative Taeniid (Ta-)TRIMs and confirmed, by using the WMISH technic, that their expression was strongly restricted to proliferative germinative cells. They concluded that Ta-TRIMs could be a good marker of germinative cells in E. granulosus.
Schistosoma spp are trematode worms that infect mammalian hosts. Eggs are released into a water source in the faeces or urine of the definitive host. The eggs hatch, releasing miracidia that infect aquatic snails. Once there, the parasite develops into a sporozoite and produces cercariae. These are released into the water and penetrate the skin of the definitive host. The parasite then sheds its characteristic forked tail to become schistosomulae and migrates to the veins. The final venule location of the adult Schistosoma is dependant of the species. The females lay eggs that migrate through the intestines to be excreted by either urination or defecation[12].
Collins et al[13] in 2013 produced the first report on adult somatic stem cells in Schistosoma mansoni. Comparing S. mansoni to already documented worms (Planarian and Echinococcus), they investigated the possible presence of neoblast-like cells in the parasite. Using EDU labelling, they observed a proliferating population in the mesenchyme of male and female parasites. Similar genes to the ones observed in planarian neoblasts were downregulated after gamma irradiation, which were correlated with a potential stem cell population in the parasite. They confirmed the self-renewal and differentiation potential of these cells using EDU/ Bromodeoxyuridine labelling studies and noted that the Sm-fgfrA gene seemed to promote the long-term maintenance of neoblast-like cells in S. mansoni following RNA interference experiments. In order to better characterise these cell populations, they investigated gene expression following gamma radiation and performed RNA interference[14]. They identified 135 downregulated genes, most of which were involved in parasite’s surface cell populations. By focusing in more detail on a specific gene (tetraspanin, TSP-2), they observed that its expression disappeared a couple of days after stem cell population depletion. They proposed that neoblast differentiation was biased towards tegument cells, especially those expressing TSP-2+.
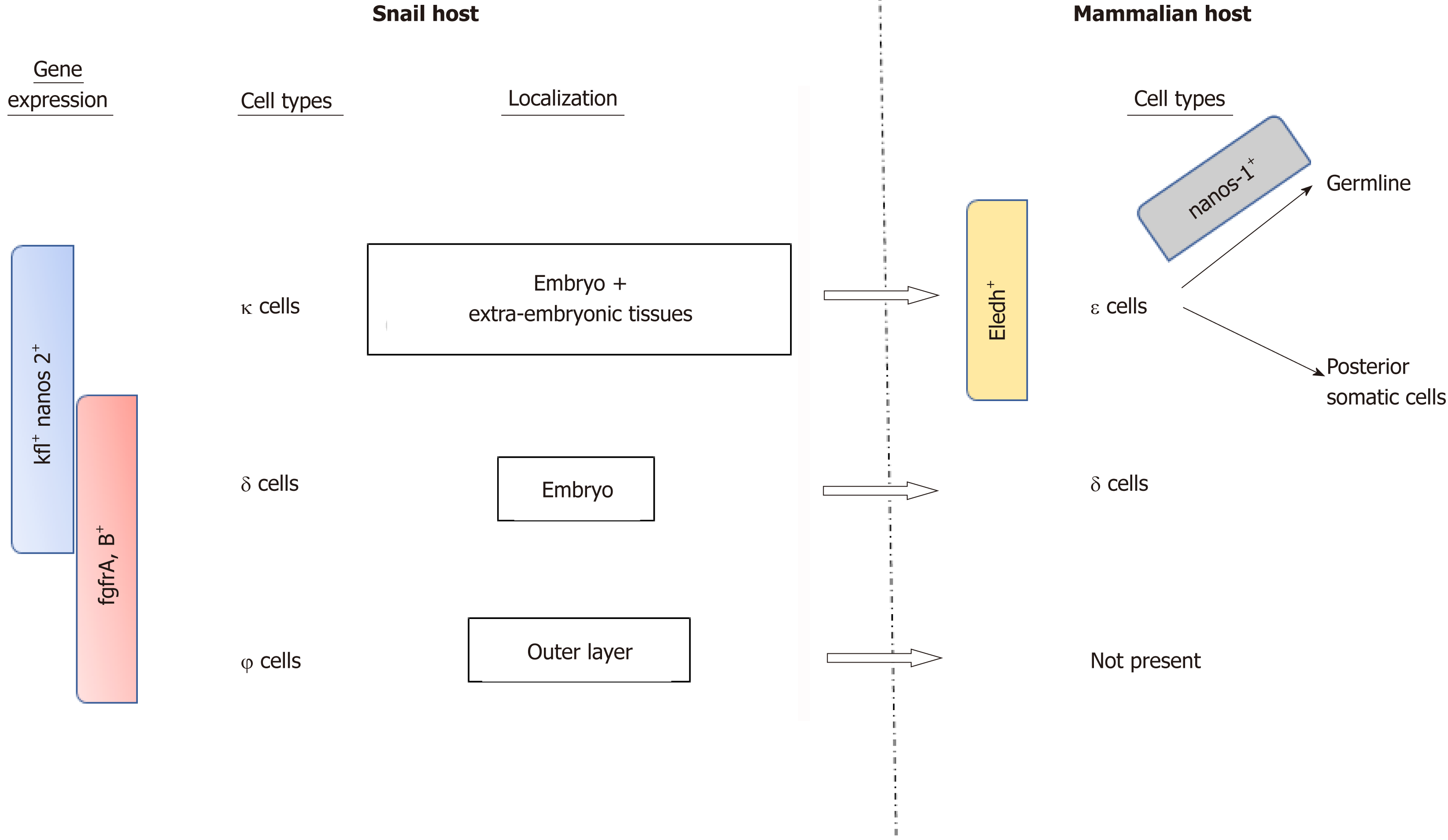
Recently, Wang et al[15] investigated the role of S. mansoni stem cells throughout the different parasite stages, including the snail hosting period (Figure 4). Using single RNA sequencing (RNA-seq) studies, they identified three distinct stem cell populations in the sporozoite stage based on the main expression of kfl nanos 2 and fgfrA, B genes: κ cells expressing kfl+ nanos 2+, ϕ cells expressing fgfrA, B+ and δ cells expression both kfl+ nanos 2+ and fgfrA, B+. During the asexual stages in the snail, δ cells were found in the embryo, as well as κ cells that were also present in extra-embryonic tissues, while the ϕ cells were mainly in the parasite’s outer layer and not in the daughter embryo. Notably, a fixed number of κ and δ cells seem to be kept by the parasite while in the mammalian host where they form the source of the previously described stem cells. Another subpopulation of stem cell derived from κ cells and expressing eledh gene (a Schistosome-specific factor) was found in juvenal parasites and named ε cells. These cells can give rise to germline or posterior somatic cells depending on the nanos-1 expression (germline if active).
In addition to the study of parasite’s own stem cells, two worm species, Planaria and Caenorhabditis elegans, although they are not or are only rarely parasitic, have served as important models for parasite stem cell and general stem cell biology, respectively, and will therefore be discussed further.
Planaria are flatworms that are only rarely parasitic. They are typically herma-phroditic but can reproduce by fission[16]. These flatworms can be compared to the parasitic trematode Schistosoma. One example of this is from Collins et al[17], who studied a bioactive peptide in the planarian Schmidtea mediterranea and used this to identify novel pro-hormones in S. mansoni.
Planarian are able to fully regenerate as they have a population of stem cells named neoblasts[18]. Rossi et al[19] identified a group of 60 genes specific to planarian stem cells using microarrays and WMISH, many of which are involved in epigenetics and regulation of transcription. Interestingly, the planarian seems to be a good model for human nervous system regeneration[20] due to the its high degree of conservation with vertebrates. Moreover, Onal et al[21] found that pluripotent genes in human stem cells were conserved in the planarian. Using fluorescence-activated cell sorting coupled with RNA-seq studies, they specifically identified octamer-binding transcription factor 4, NANOG, and sex determining region Y-box 2. This later study proved that planarian organisms are valuable models to better understand human stem cells.
C. elegans is a roundworm belonging to the nematode family. This organism is well known by scientists as it is one of the most studied and best models for fundamental research as summarised in Kevin Strange’s review[22]. It has been extensively used as a parasite model[23,24]. A better understanding of C. elegans stem cell biology would allow a better understanding of stem cell biology in general[25]. Among the many studies involving C. elegans stem cells, we can cite the work from Seidel et al[26] who studied stem cell quiescence following starvation, or Noormohammadi et al[27] who showed that TRiC/CCT assembly is linked to pluripotency of human stem cells and resistance to proteotoxic stress in C. elegans.
Parasite stem cells are evidently an interesting topic that is becoming increasingly better studied. Although their strong regeneration capacity is beyond that of their human counterparts, they serve to facilitate our understanding of stem cell capabilities and biology in general.
Parasites invade and occupy a number of different niches within their host species. Consequently, they interact with stems cells in a variety of ways. Many of the interactions are indirect due to tissue damage caused by the parasite, which trigger host immunity and damage repair mechanisms. Other interactions can be more direct whereby the parasite or soluble parasitic factors directly affect stem cell proliferation. An additional layer of complexity is the ability of certain parasites to manipulate their host by not only inducing stem cell proliferation, but also stimulating differentiation into the parasite’s preferred cell type. Evidence of such interactions will be discussed below.
As well as infecting humans and other mammals, various insect species are subject to parasitism. In many cases, insects serve as disease vectors transmitting the parasite from one human to another. The parasites often go through developmental stages within their host insect and therefore impact insect fitness and insect stem cell biology.
Mosquitos: The malaria parasite Plasmodium uses female Anopheles mosquitos, which feed on mammalian blood as an intermediate host. Upon consuming a blood meal, the parasite enters the mosquito midgut. Following transversal of the midgut epithelium, the parasite undergoes a multiplication phase and the progeny migrate to the mosquito salivary gland. These parasites are then injected into another human during a subsequent blood meal. During invasion and intracellular migration of the mosquito midgut epithelium, the parasite triggers apoptosis and necrosis of invaded cells[28]. Surprisingly, this is not detrimental to mosquito survival. During infection of Anopheles stephensi with Plasmodium falciparum, Baton and Ranford-Cartwright (2007) showed that midgut regenerative cells underwent proliferation and differentiation to replace columnar midgut cells. Moreover, the number of proliferating and differentiating cells correlated with the level of midgut cell destruction. This indicates that the mosquito is able to compensate for parasitic damage through proliferative regeneration, inadvertently permitting parasite transmission[29].
Honey bees: A similar protective mechanism to that described for the mosquito was predicted in the honey bee, Apis mellifera. The honey bee endures enterocyte damage during infection with the parasitic microsporidian, Nosema ceranae[30]. Despite reports elsewhere that G1/S phase genes were upregulated during N. ceranae infection[31], Panek et al[32] recently showed a reduction in the replicative capacity of intestinal stem cells during infection. Drosophila infection with the parasitic wasp Leptopilina boulardi induces haematopoiesis through ROS-dependent induction of the Toll/NF-KB and EGFR pathways. L. boulardi also stimulates the preferential differentiation of lamellocytes, increasing their proportion to nearly 50% of all haemocytes. These cells are specifically involved in pathogen encapsulation as well as killing and implicate the existence of a “do or die” mechanism, as survival depends on the neutralisation of wasp eggs before they hatch[33]. Evidently, insects have utilised their stem cell response when necessary to permit endurance or destruction of the parasite insult.
Helminths: Trichinella spiralis is a nematode parasite that infects many carnivorous and omnivorous animals, including humans, and can be acquired by ingesting cysts from contaminated/undercooked meat[34]. Infection of Balb/c mice with parasitic worms such as T. spiralis or Heligmosomoides polygyrus (a mouse model for human helminth infections) have been shown to induce changes in stem cell proliferation within intestinal crypts of their hosts. T. spiralis leads to small intestine inflammation and an initial increase in epithelial stems cells (Ki-67-positive) and/or transient daughter cells (day 2 post infection). This alters the architecture of the intestinal crypt and causes an upward shift in the proliferative zone[35]. In another nematode parasite, Trichuris muris, such alterations are considered to facilitate expulsion of the parasite from its inter-epithelial niche[36]. However, during T. spiralis infection, the proliferative response is not sustained. The Ki-67-positive population decreased by days 6-12 post-infection, while the number of secretory Paneth cells increased (possibly due to differentiation). This may be advantageous as secretory products from Paneth cells, such as antimicrobial peptides and proteins, are considered important for host protection against luminal microorganisms[35]. Conversely, adult intestinal stem cells were shown to be repressed in Balb/c mice infected with H. polygyrus. During infection, granuloma associated crypts exhibited loss of the adult intestinal stem cell markers Lgr5-GFP and Olfm4, while Ly6a (which encodes stem cell antigen 1 (Sca-1)), was upregulated. Nippostrongylus brasiliensis, a nematode parasite that does not invade intestinal tissue, did not induce Sca-1. This suggests that upregulation of Sca-1 is a specific response to crypt injury induced by H. polygyrus. Immune cell-derived interferon gamma (IFNγ) was critical for the granuloma associated crypt response. Interestingly, foetal stem cell markers Gjal and Spp1 were shown to be upregulated in the granuloma associated crypt, implicating a novel mechanism to repair intestinal crypt disruption.
Toxoplasma: Toxoplasma is a single-cell, protozoan parasite and is the causative agent of toxoplasmosis. Most human infections are asymptomatic, but the disease is more severe in immune compromised individuals and infants, if the mother were to become infected during pregnancy. Cats are the definitive host and shed the oocysts in their faeces which can be accidentally ingested by humans and other mammals or birds[37]. T. gondii induces apoptosis and inhibits the differentiation of neuronal stem cells. These interactions are considered major contributors to congenital neuro-pathology. Various markers of apoptosis were found to be upregulated during the co-culture of T. gondii tachyzoites and neuronal stem cells. These experiments used a trans-well culture system, which only permits the passage of parasitic soluble factors, not the parasite itself. Consequently, inhibition was attributed to T. gondii excreted-secreted antigens (Tg-ESAs). The disruption of endoplasmic reticulum homeostasis occurs in many diseases and can lead to the activation of cell death pathways. Wang et al[38] showed decreased apoptosis following pre-treatment with the endoplasmic reticulum stress inhibitor TUDCA, implicating the involvement of the endoplasmic reticulum stress pathway in the interaction between Tg-ESAs and neuronal stem cell apoptosis. Tg-ESAs were also found to inhibit the differentiation of neuronal stem cells into neurones and astrocytes. This was determined by measuring the concentration of cell markers, specifically βIII-tubulin and glial fibrillary acidic protein. These decreased in a dose-dependent manner. A reduction in β-catenin and interactivity effects between Tg-ESAs and wnt3a (an activator of the Wnt/β-catenin pathway) implicated the involvement of the Wnt/β-catenin pathway, an important differentiation pathway in neuronal stem cells[39]. Further work implicated the virulence factor Rhoptry protein 18 as a partial mediator of the endoplasmic reticulum stress-induced apoptosis and cell differentiation (Wnt/β-catenin) pathways[40].
Plasmodium: Once injected into the human host via the bite of the female Anopheles mosquito, the parasites invade hepatocytes and multiply, forming liver schizonts[41]. These burst releasing merozoites, which invade erythrocytes, and begin the asexual reproductive cycle. During early malarial infection, both MSCs and HSCs are produced in the bone marrow (BM) and subsequently migrate to the spleen. Interestingly, adoptive transfer of P. berghei-induced MSCs conferred resistance to P. berghei in naive mice. MSCs were shown to migrate to the spleen, enhance pro-inflammatory cytokine production (specifically interleukin 12 [IL-12]), inhibit anti-inflammatory cytokines (specifically IL-10), and inhibit the accumulation of T-regulatory cells, which are induced by malaria as an immune evasion mechanism. Adoptive transfer of MSCs in late stage infections, however, did not alter disease progression[42]. An early haematopoiesis response is considered protective to the host, to counteract erythrocyte loss caused by parasite maturation in red blood cells. Unfortunately, the response cannot sustained during the course of malaria infection leading to the common disease-associated pathophysiology of anaemia[43]. One suggestion is that there is competition for the production of erythropoietic and immune cell, both derived from pluripotent stem cells[44]. Interestingly, during low oxygen conditions the kidneys produce erythropoietin, which in turn stimulates erythropoiesis. During mouse infection with P. vinckei or P. chabaudi, but not P. berghei, further erythropoiesis could be stimulated by exposing infected mice to high altitude conditions (hypoxia) in a decompression chamber. This indicated that the infection with P. berghei had already saturated the maximal erythropoiesis response. The initial increase in HSCs during early infection may be due to P. berghei’s preference for invasion of reticulocytes. However, cellularity (total number of nucleated cells) and subsequently erythropoietic capacity in the BM decreased during the course of malaria infection. Despite enlargement and an increase in cellularity, the spleen does not compensate to meet the erythropoietic and hemopoietic needs of the host, which leads to anaemia. Although there are reports that pluripotent stems cells remain in G0 and are therefore protected, the lethality and severity of anaemia during malaria infection may be determined by irreversible damage to the self-renewal capacity of hematopoietic stems cells, as observed with chronic leishmania infection[45].
Leishmania: Leishmania spp have a broad spectrum of interactions with various host stem cell lineages. Although macrophages are their primary host, they have been shown to be capable of infecting a variety of cells including different types of stems cells both in vitro and in vivo[46-49]. Allahverdiyev et al[47] showed in vitro invasion of adipose tissue-derived mesenchymal stem cells by L. donovani, L. major, L. tropica, and L. infantum parasites, raising potential concerns about stem cell transplantations and parasite transmission, which is discussed in more detail later. BM-derived MSCs (BM-MSCs) were also shown to be vulnerable to L. infantum and L. donovani invasion in an in vivo mouse model. This was confirmed by confocal microscopy and co-staining with antibodies for parasites and MSCs (CD271+/Sca1+) isolated from both the BM and spleen of infected mice. Interestingly, the authors not only visualised this invasion events ex vivo, but determined the viability of the amastigotes identified in BM-MSC CD27+CD45 cells by inoculating the infected cells into an axenic in vitro culture system. Then promastigote forms were detected. Since MSCs have potent drug efflux pumps, the proposed purpose of this interaction is the potential avoidance of drug-induced death. This is considered particularly important for latent infections that occur despite treatment. Such stem cells niches could also serve as a “hide-out” for parasites during asymptomatic infections.
As well as the modulation of host stem cell proliferation, following the direct invasion of stem cells, tissues infected with Leishmania parasites have also been reported to display alterations in host cytokine/chemokine expression and stem cell proliferation. Dameshghi et al[50] in 2016 isolated macrophages and adipose-derived MSCs from mice that were either sensitive or resistant to Leishmania. Isolated cells were placed, in various combinations, in a trans-well system. After 72 h of co-culture the MSC chamber was removed and the macrophage supernatant was replaced before challenge with L. major promastigotes. These macrophages, now considered MSC-educated, showed a reduction in phagocytosis, nitric oxide, IL-10, tumour necrosis factor alpha production and an induction in inflammatory cytokines in response to L. major infection. This response was independent of prior sensitivity to Leishmania (although the magnitude of the response did vary between treatment-group combinations). This implicates MSCs as the sensors and switchers of immune modulation and supports the potential of stem cells as a treatment for leishmaniasis. Local alterations in hematopoietic activity have also been reported in tissues harbouring L. donovani amastigotes in vivo[51]. Infection leads to an increase in progenitor cell frequency in the blood, and a correlation was observed between hematopoietic activity and parasite proliferation in the spleen and BM. Tissue-specific regulation of cytokine and chemokine expression was also observed, with an increase in those associated with monocyte and granulocyte maturation from multipotential precursors. Leishmania-infected stromal cells also display an enhanced capacity in vitro, through cytokine regulation, to support the differentiation of regulatory dendritic cells from hematopoietic progenitor cells[52]. The recombinant heat shock protein 70, from a related kinetoplastid parasite Trypanosoma cruzi, was also shown to have an immune-stimulatory effect on the maturation of dendritic cells from BM precursors. Remarkably, dendritic cell maturation occurred in the presence of the recombinant protein alone, fused to the KMP11 antigen as well as a 242-amino acid protein fragment[53]. This again could have implications for therapy. As the main source of immune cells, it is not surprising that BM-hematopoietic stem/progenitor cells are activated during Leishmania infection. This is consistent with the stress response to other acute infections[54]. However, L. donovani was shown to be capable of host manipulation by skewing HSC expansion to the differentiation of non-classical myeloid progenitor cells. Interestingly, cells produced in this manner were not only the preferred target of the parasite but were also more permissive to Leishmania infection, providing evidence of parasite manipulation[54]. Acute infections can give rise to such expansion that cannot be sustained during chronic infection. Using adoptive transfer of IFNγ sufficient CD4+ T cells into immune-deficient mice, Pinto et al[55] in 2017 showed that chronic stimulation of HSC expansion, by this pathway, leads to hematopoietic exhaustion. In this instance, the reservoir of long-term HSCs irreversibly loses quiescence and enters into an active cell cycle, where they eventually lose their self-renewal capacity. During chronic infections, an effective anti-parasitic response is therefore at the expense of the reservoir of quiescent LT-HSC hematopoietic fitness. The importance of this trade-off for sufferers of life-long chronic infections is yet to be determined.
Evidently, parasites can develop a range of complex interactions with their host’s stem cells by modulating their expression and fate. Although the growing body of research into this area in recent years (Figure 3) has meant that we know more now than ever before, the field remains in its infancy. Most of the aforementioned studies were conducted in insect or mouse models, as obvious technical and ethical issues limit human in vivo investigations. Due to the complexity of parasite life cycles, often having distinct development stages in multiple host tissues, studies into general parasite biology during human infection are also limited by the in vitro culture systems we have available. However, as our in vitro capabilities for stem cells production have expended parasitologist can utilise stems cells in order to recreate and study parasite development within a variety of host tissues that were previously inaccessible. Examples of such studies are detailed in Table 1. The availability of these valuable model systems will not only permit a better understanding of parasite biology but may open up new avenues for parasite treatment.
| Stem cell type | Parasite | Details | Future purpose/possibilities | Ref. |
| Hematopoietic | Plasmodium vivax | Cluster of differentiation 34+ derived reticulocytes | Growing Plasmodium species that have a reticulocytes preference | Grosgogeat et al[100] |
| Leishmania infantum | BM HSCs-derived white cells were capable of phagocytosing and supporting promastigote development | Better understanding of promastigote development within macrophages | Carvalho-Gontijo et al[101] | |
| Neuronal | Toxoplasma gondii | Fibroblast-derived and CD34+-derived human neuronal-like cells were capable of supporting T. gondii tachyzoite development | Better understanding of parasite behaviour and development within human brain cells | Passeri et al[102], Tanaka et al[103] |
| Liver cells | Plasmodium falciparum, vivax, yoelii, and berghei | Human iPSC-derived hepatocyte-like cells, expressing important Plasmodium entry receptors CD81 and the SRB1, were permissive to the development many Plasmodium species | Better understanding of liver stage infection. Particularly for species such as Plasmodium vivax that can remain dormant in the liver and recrudesce many years later | Teranishi et al[104] |
Stem cell therapies have become increasingly more applied as potential cures for important diseases such as liver injuries, muscle degeneration or anaemia[56]. These therapies aim to replace dysfunctional cells or tissues with the transplantation of newly generated and functional stem cells. In addition to these applications, a new field of use for stem cell therapy has recently emerged: Parasitic treatment. The aim of these treatments is not to directly target the parasite itself but to help the patient fight and recover post infection, as we will discuss further in this section.
The first parasite infection that we will cover is the kinetoplastid parasite T. cruzi. This parasite is responsible for Chagas disease, that affects up to 7 million people, and predominantly occurs in Latin America[57]. The disease is curable if treated during early stage of infection, however treatment efficacy decreases with time. There are two distinct infection stages: Acute and chronic. The former stage can last up to 2 wk and, though the patient may have a high parasitaemia, the symptoms remain relatively mild and non-specific[58]. The latter stage can be subdivided into determi-nate and indeterminate phases. The determinate phase is marked by an equilibrium stage where host and parasites co-exist with no damage. Following an unknown mechanism, up to 30% of the patients will switch to the indeterminate phase. During this stage, Chagas disease-associated severe complications such as gastrointestinal or chronic heart diseases occur. Chagas heart disease remains the most severe and prevalent complication (45% of the chronic patients), which explains why it has been more extensively described. It is characterised by an extensive fibrosis of the myocardium that can evolve into chronic heart failure[59]. The treatment for Chagas heart disease cases are symptom dependent but can eventually lead to a heart transplant. This has further associated consequences and due to immunosuppression could lead to the reactivation of the parasitic infection[60]. It is thus very important to find an alternative method to repair cardiac tissue affected by the parasite. Soares et al[61] in 2004 were the first to use MSCs to treat heart failure following a parasitic infection. They transplanted BM of a healthy mouse into a mouse model of Chagas heart disease and observed a reduction in inflammation and fibrotic areas by 80% and 90% respectively. Interestingly, BMC treatment was shown to affect the myocardium transcriptome by decreasing the expression of upregulated genes involved in inflammation and fibrotic events in T. cruzi-infected mice[62]. One striking example is the strong downregulation of Galectin-3. Galectin-3 is a protein that is expressed by macrophages and correlates with inflammation in the mouse model of Chagas heart disease via its involvement in the induction of collagen production, proliferation of cardiac fibroblast and T-cell apoptosis suppression. This work implicates Galectin-3 as a potential therapeutic target and highlights the possible use of BMC injection as anti-inflammatory and anti-fibrotic treatment of Chagas’s heart disease.
Following the initial success with mouse-models (reviewed by Carvalho et al[63]) the next step was to evaluate whether such results could be achieved during human infection. The first clinal trial took place on a 52-year-old man suffering of heart failure due to T. cruzi infection[64]. Purified mononuclear cells, isolated from healthy BM, were injected into the patient and an improved ventricular function was observed 30 d post-transplantation. This first success lead to additional studies which further expanded to a multi-centre randomised clinical trial. Over a total of 183 patients were treated with BM mononuclear cells in conjunction with granulocyte-colony stimulating factor. Unfortunately, no improvement in cardiac function was observed[65]. Although the results with the BM-derived mononuclear cells were not as good as expected, the use of a different type of stem cells, MSCs, did yield some interesting results and showed immunomodulatory properties as well as the potential for use in chronic chagasic cardiomyopathies[66]. Notably, only a small fraction of injected MSCs actually migrated to the heart in a Chagas mouse model. Indeed, nearly 70% could be found in liver, lungs, and spleen. Nevertheless, a reduction of the right ventricular dilatation was observed after MSC transplantation in Chagas mouse models. A further study showed that MSC treatment could influence gene expression and more especially the extracellular matrix protein laminin γ1 that was upregulated in infected mice compared to the MSCs treated ones[67]. Interestingly, two antagonist factors (the pro-inflammatory INFγ and the anti-inflammatory IL-10) that were upregulated in infected mice are down-regulated after MSC treatment. The pro-inflammatory stromal cell-derived factor-1 gene was also upregulated after MSC treatment, which could be correlated to the attraction of additional stem cells to the damaged area. More recently, Larocca et al[68] did not observe any cardiac function improvements following adipose-derived MSCs but a decrease in fibrosis and inflammatory cells. Evidently, there has been some difficulty in defining effective stem cell therapeutic targets, and further translating these from Chagas heart disease mouse models to the human disease system, but the value of such a treatment warrants further research into this area.
Leishmaniasis is a parasitic infection that can take different forms dependent on the parasitic strain. Three distinct forms affect different targets: the cutaneous form (that can be caused by almost any leishmania spp) affects the skin with the presence of lesions, the mucocutaneous form (caused by L. Donovani and L. infantum) leads to a partial or full destruction of mucosal membranes around the mouth, nose or throat while the visceral form affects the liver, BM, and spleen[69]. Leishmaniasis symptoms can also be due to an alteration of the host immune response. Knowing that MSCs can modulate the immune response[70], it seems reasonable to use these cells as therapy against cutaneous leishmania as Pereira et al[71] investigated. Unfortunately, they did not observe any protective effect, post-MSC injection, in a mouse model of cutaneous infection and even reported an increase in parasitic burden dependent on the route of MSC administration. This increase was correlated to an increase of IL-10 producing CD4+ and CD8+ T cells in the spleen. Of note, Il-10 is an anti-inflammatory cytokine that can limit the ability of macrophages to kill intracellular organisms and the lack of T-cell-derived IL-10 enhances protection from L. major infection in mouse as previously described[72]. Besides cutaneous leishmaniasis, stem cell therapies could be applied to other Leishmania strains such as the visceral and mucocutaneous forms of the disease. Studying L. major (responsible for the visceral form), Dameshghi et al[50] obtained promising immunomodulatory results from a cell-based assay, nevertheless the results still have to be translated to an in vivo system.
Schistosomiasis is caused by a parasitic trematode. These worms remain in the host’s blood vessels where the female lays the eggs that are the primary cause of pathology during chronic disease[73]. Proteolytic enzymes released by the eggs trigger a T-helper type 2 response that can lead to the fibrosis of affected tissues[74]. There are two main types of schistosomiasis infection: Urogenital schistosomiasis caused by S. haemato-bium and hepatic schistosomiasis caused by S. mansoni, S. intercalatum, S. japonicum, and S. mekongi[75]. The urogenital schistosomiasis can lead to fibrosis of the bladder and lower ureters and can even evolve to kidney failure. The hepatic schistosomiasis shows two distinct forms: Fibrotic hepatic disease and early inflammatory. The fibrotic hepatic disease (Symmer’s pipestem fibrosis) is caused by the deposition of collagen in the periportal spaces, leading to the tissue’s hypoxia. Studies have already been conducted to investigate the potential of MSCs to cure these symptoms and a decrease of liver fibrosis has been observed in treated mouse models of S. mansoni[76]. Moreover, El-Shennawy et al[77] recently showed that a treatment with BM-MSCs significantly decreased the granulomas size and the expression of alpha-smooth muscle actin (a fibrosis factor) in hepatic stellate cells. The anti-fibrotic effect of the MSCs was not limited to these features and it included the inhibition of collagen deposition, a downregulation of transforming growth factor β1 and an upregulation of matrix metalloprotease 9, a matrix metalloprotease that leads to hepatic stellate cells apoptosis. As hepatic stellate cells are involved in the hepatic fibrosis process during liver injury[78], early injection of MSCs to trigger their apoptosis may lead to a more efficient cure. MSCs have also been used to treat S. japonicum mouse models using single injections or in combination with praziquantel (current drug treatment against schistosomiasis) and a recovery of the spleen and liver was observed due to the inhibition of the collagen deposition[79]. Another stem cell source has been tested by Elkhafif et al[80] in 2011 who used CD133+ human umbilical cord blood stem cells to trigger the production of new blood vessels in order to promote vascularisation. This neo-vascularisation created a permissive environment allowing better survival of damaged cells.
The Plasmodium parasite, the causative agent of malaria, is an important public health threat. Symptoms of the infection are initially non-specific and include headache, fever and fatigue[81]. How the infection progresses will then be defined as uncomplicated or severe (complicated) malaria. The severe form can be fatal with symptoms including consciousness, convulsions, bleeding, anaemia, renal impair-ment and pulmonary oedema[82]. The erythrocyte is one niche exploited by parasite in the human host and during a 48 or 72 h erythrocytic cycle (dependent on the species) the parasite continuously occupies, destroys and invades yet more red blood cells, thus having a huge impact on blood cell homeostasis. Knowing this feature, the use of HSC could be an interesting therapeutic approach. Indeed, Belyaev et al[83] in 2010 identified, in the BM of P. chabaudi infected mice, a new subset of haematopoietic progenitors triggered by IFNγ signal. The injection of these healthy atypical HSC progenitors within infected mice showed a greater clearance of infected erythrocytes leading to a decrease in infection-associated anaemia. MSCs isolated from the spleen of P. berghei infected mice, could be transplanted to naïve mice prior their exposition to P. berghei[42]. Interestingly, these MSCs produced protective cytokines such as tumour necrosis factor alpha and IL-1β that decreased the production of haemozoin (product from the erythrocyte haem degradation). This helps to counteract host evasion mechanisms used by the parasite as hemozoin inhibits macrophages and decreases the number of Treg cells in the spleen[84]. These cells can be used to treat cerebral malaria, a severe complication of P. falciparum infection, characterised by neurological symptoms including coma, convulsion, drowsiness and confusion and can affect spleen, kidneys and lungs[85]. Treatment of BM-MSCs improved the clearance of parasitised erythrocytes, increased the regeneration of hepatocytes and Kupffer cells (specific phagocytic cells) and increased the number of astrocytes and oligodendrocytes in the brain. In parallel, lung inflammation was reduced, by limiting collagen deposition, and the number of neutrophils, and amount of malaria pigmentation was decreased while the mesangial architecture of the kidney was restored[86]. Despite all these improvements, no reduction of the brain damage was observed.
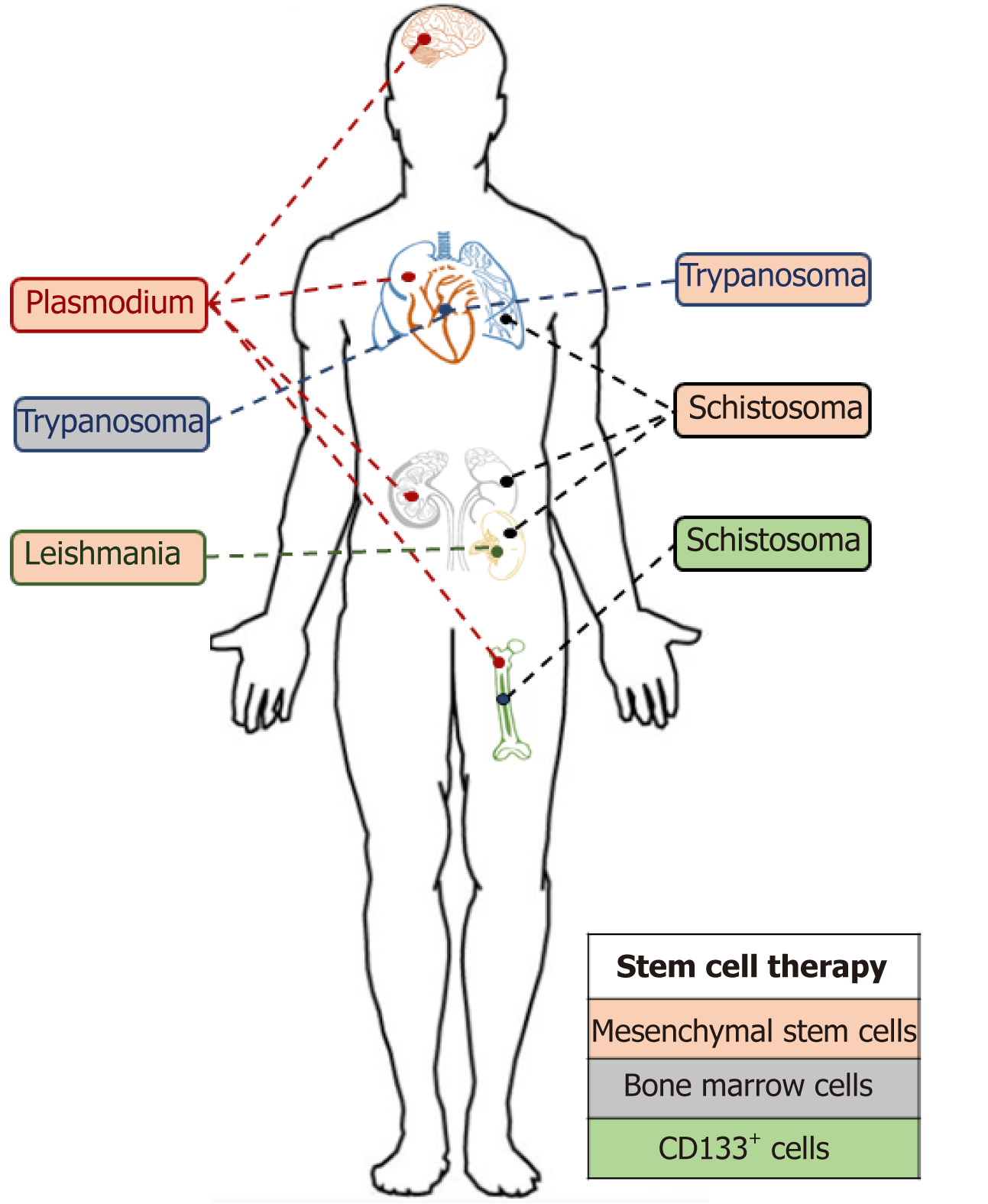
Stem cell therapies have the potential to significantly alter the way in which we treat parasitic infections (Figure 5). The transplantation of stem cells to aid the recovery of damaged organs remains a really interesting addition to traditional parasitic cure methods. However, these methods and techniques are still in their infancy, and the affordability of stem cell-based therapies will be a significant factor in their uptake, particularly in developing countries where these neglected tropical diseases predominate[87].
The future availability of stem cell therapies were predicted to be revolutionary for the cure of multiple disease as emphasised by the recent increase in interest in this research area and the number (7150 with the term “stem cells”) of clinical trials registered with clinicaltrials.gov. Nevertheless, only HSCs have been so far approved by United States Food and Drug Administration to serve as cure therapy[88]. They are mainly used, through BM transplantation, to cure blood disorders such as leukaemia or myeloma. Prior to any transplantation, donations are screened for any potential infection. Some screens such as HIV 1+2, hepatitis C, hepatitis B, syphilis, human T-lymphotropic virus 1+2 are mandatory in United Kingdom[89]. However cytomegalovirus, malaria, Trypanosoma cruzi and West Nile virus remain simply additional. These additional screens are only performed if a donor risk has been identified. Multiple parasitic infections have been identified in recipient’s post-transplantation (as discussed below). However, the immunosuppressive medication following an allogenic transplantation makes the patient more sensitive to opportunistic parasitic infections and must be considered as a potential source/ route of infection without the availability of donor pre-screens.
Though Leishmaniasis does not appear on the list of infectious pathogens screened prior to transplantation, its ability to survive within an intracellular environment by creating a suitable niche for replication[90] remains a threat. Indeed, although the parasite primarily invades phagocytic cells, i.e. macrophages, the amastigote form has been found in fibroblasts[48], hepatocytes[49] and amniotic epithelial cells[91]. The potential of Leishmania to survive in MSC has been recently investigated by Allahverdiyev et al[47] who were able to observe an in vitro infection of adipose-derived MSCs by L. tropica, L. donovani, L. major and L. infantum.
Of note, several cases of leishmania infections post MSC transplantations have been documented[92,93] though the presence of the parasite in the transplant was not proved. Several hypotheses could be raised, i.e. an infectious sand fly bite post transplantation, a latent infection in the patient or a re-activation of the opportunist parasite due to the immunosuppressive treatment. The Plasmodium parasite has also been described in the BM of an infected individual[94] and has been reported to be capable of invading HSC progenitors[95]. Moreover, the parasite, once in the human host, remains in the liver for 7 to 10 d and sometimes for a longer period as the hypnozoite form. For these reasons, reports of malaria infection following transplantation are not surprising. Indeed, Martín-Dávila et al[96] recently reported patients infected by P. malariae and P. ovale after liver and kidney transplantations. Parasite transmission can also occur after BM transplantation as described in a recent case even when the donor was not classified at risk[97].
Toxoplasma is a parasite that does not appear on the list of pathogen screens prior to transplantation as they are unlikely to be found in stem cells. Nevertheless, several cases of toxoplasmosis infected patients post-HSC transplantation have been described[98] mostly as an opportunistic infection following the immunosuppression treatment post-transplantation. Another parasite taking advantage of the lower immune defence post stem cell injection is cryptosporidium, which has also been identified in patients post transplantation[99].
It is thus necessary to consider parasites during the screening process pre-transplantation and not only based on donor recent travel history to endemic areas. The follow up post-transplantation monitoring should also not be neglected as opportunistic infections represent an important threat.
As can be seen from this review, the two seemingly distinct fields of stem cell research and parasitology are in fact closely entwined. Not only do multicellular parasites have stem cells of their own, but certain parasites exploit stem cells to create niches either within their host stem cells, or by modulating host stem cell activity to create a more habitable environment. The use of stem cells as therapies to treat either the parasitic diseases or, more commonly, the sequalae associated with the infection, is becoming increasingly more studied. The presented literature allows us to highlight the importance of considering stem cells as important targets for parasites and not underestimate this relationship from a clinical point of view.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu L S-Editor: Zhang L L-Editor: Filipodia E-Editor: Xing YX
| 1. | He S, Nakada D, Morrison SJ. Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol. 2009;25:377-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 404] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 2. | Ulloa-Montoya F, Verfaillie CM, Hu WS. Culture systems for pluripotent stem cells. J Biosci Bioeng. 2005;100:12-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Costamagna D, Berardi E, Ceccarelli G, Sampaolesi M. Adult Stem Cells and Skeletal Muscle Regeneration. Curr Gene Ther. 2015;15:348-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Centers for Disease Control and Prevention. About Parasites. Available from: https://www.cdc.gov/parasites/about.html. |
| 5. | Poulin R, Morand S. The diversity of parasites. Q Rev Biol. 2000;75:277-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 350] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Shiadeh MN, Niyyati M, Fallahi S, Rostami A. Human parasitic protozoan infection to infertility: a systematic review. Parasitol Res. 2016;115:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | World Health Organization. Global health estimates 2016: Deaths by cause, age, sex, by country and by region, 2000-2016. 2018; World Health Organization. |
| 8. | Maehle AH. Ambiguous cells: the emergence of the stem cell concept in the nineteenth and twentieth centuries. Notes Rec R Soc Lond. 2011;65:359-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Centers for Disease Control and Prevention. Parasites - Echinococcosis. Available from: https://www.cdc.gov/parasites/echinococcosis/index.html. |
| 10. | Koziol U, Rauschendorfer T, Zanon Rodríguez L, Krohne G, Brehm K. The unique stem cell system of the immortal larva of the human parasite Echinococcus multilocularis. Evodevo. 2014;5:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Koziol U, Radio S, Smircich P, Zarowiecki M, Fernández C, Brehm K. A Novel Terminal-Repeat Retrotransposon in Miniature (TRIM) Is Massively Expressed in Echinococcus multilocularis Stem Cells. Genome Biol Evol. 2015;7:2136-2153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Centers for Disease Control and Prevention. Parasites - Schistosomiasis. Available from: https://www.cdc.gov/parasites/schistosomiasis/biology.html. |
| 13. | Collins JJ, Wang B, Lambrus BG, Tharp ME, Iyer H, Newmark PA. Adult somatic stem cells in the human parasite Schistosoma mansoni. Nature. 2013;494:476-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 14. | Collins JJ, Wendt GR, Iyer H, Newmark PA. Stem cell progeny contribute to the schistosome host-parasite interface. Elife. 2016;5:e12473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Wang B, Lee J, Li P, Saberi A, Yang H, Liu C, Zhao M, Newmark PA. Stem cell heterogeneity drives the parasitic life cycle of Schistosoma mansoni. Elife. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Malinowski PT, Cochet-Escartin O, Kaj KJ, Ronan E, Groisman A, Diamond PH, Collins ES. Mechanics dictate where and how freshwater planarians fission. Proc Natl Acad Sci U S A. 2017;114:10888-10893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Collins JJ, Newmark PA. It's no fluke: the planarian as a model for understanding schistosomes. PLoS Pathog. 2013;9:e1003396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Baguñà J. The planarian neoblast: the rambling history of its origin and some current black boxes. Int J Dev Biol. 2012;56:19-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Rossi L, Salvetti A, Marincola FM, Lena A, Deri P, Mannini L, Batistoni R, Wang E, Gremigni V. Deciphering the molecular machinery of stem cells: a look at the neoblast gene expression profile. Genome Biol. 2007;8:R62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Gentile L, Cebrià F, Bartscherer K. The planarian flatworm: an in vivo model for stem cell biology and nervous system regeneration. Dis Model Mech. 2011;4:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Onal P, Grün D, Adamidi C, Rybak A, Solana J, Mastrobuoni G, Wang Y, Rahn HP, Chen W, Kempa S, Ziebold U, Rajewsky N. Gene expression of pluripotency determinants is conserved between mammalian and planarian stem cells. EMBO J. 2012;31:2755-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Strange K. An overview of C. elegans biology. Methods Mol Biol. 2006;351:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Gilleard JS. The use of Caenorhabditis elegans in parasitic nematode research. Parasitology. 2004;128 Suppl 1:S49-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Burns AR, Luciani GM, Musso G, Bagg R, Yeo M, Zhang Y, Rajendran L, Glavin J, Hunter R, Redman E, Stasiuk S, Schertzberg M, Angus McQuibban G, Caffrey CR, Cutler SR, Tyers M, Giaever G, Nislow C, Fraser AG, MacRae CA, Gilleard J, Roy PJ. Caenorhabditis elegans is a useful model for anthelmintic discovery. Nat Commun. 2015;6:7485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 25. | Joshi PM, Riddle MR, Djabrayan NJ, Rothman JH. Caenorhabditis elegans as a model for stem cell biology. Dev Dyn. 2010;239:1539-1554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Seidel HS, Kimble J. Cell-cycle quiescence maintains Caenorhabditis elegans germline stem cells independent of GLP-1/Notch. Elife. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Noormohammadi A, Khodakarami A, Gutierrez-Garcia R, Lee HJ, Koyuncu S, König T, Schindler C, Saez I, Fatima A, Dieterich C, Vilchez D. Somatic increase of CCT8 mimics proteostasis of human pluripotent stem cells and extends C. elegans lifespan. Nat Commun. 2016;7:13649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Aly AS, Vaughan AM, Kappe SH. Malaria parasite development in the mosquito and infection of the mammalian host. Annu Rev Microbiol. 2009;63:195-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | Baton LA, Ranford-Cartwright LC. Morphological evidence for proliferative regeneration of the Anopheles stephensi midgut epithelium following Plasmodium falciparum ookinete invasion. J Invertebr Pathol. 2007;96:244-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Paris L, El Alaoui H, Delbac F, Diogon M. Effects of the gut parasite Nosema ceranae on honey bee physiology and behavior. Curr Opin Insect Sci. 2018;26:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Martín-Hernández R, Higes M, Sagastume S, Juarranz Á, Dias-Almeida J, Budge GE, Meana A, Boonham N. Microsporidia infection impacts the host cell's cycle and reduces host cell apoptosis. PLoS One. 2017;12:e0170183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Panek J, Paris L, Roriz D, Mone A, Dubuffet A, Delbac F, Diogon M, El Alaoui H. Impact of the microsporidian Nosema ceranae on the gut epithelium renewal of the honeybee, Apis mellifera. J Invertebr Pathol. 2018;159:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Louradour I, Sharma A, Morin-Poulard I, Letourneau M, Vincent A, Crozatier M, Vanzo N. Reactive oxygen species-dependent Toll/NF-κB activation in the Drosophila hematopoietic niche confers resistance to wasp parasitism. Elife. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Centers for Disease Control and Prevention. Parasites - Trichinellosis (also known as Trichinosis). Available from: https://www.cdc.gov/parasites/trichinellosis/biology.html. |
| 35. | Walsh R, Seth R, Behnke J, Potten CS, Mahida YR. Epithelial stem cell-related alterations in Trichinella spiralis-infected small intestine. Cell Prolif. 2009;42:394-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 328] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 37. | Centers for Disease Control and Prevention. Parasites - Toxoplasmosis (Toxoplasma infection). Available from: https://www.cdc.gov/parasites/toxoplasmosis/. |
| 38. | Wang T, Zhou J, Gan X, Wang H, Ding X, Chen L, Wang Y, DU J, Shen J, Yu L. Toxoplasma gondii induce apoptosis of neural stem cells via endoplasmic reticulum stress pathway. Parasitology. 2014;141:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Gan X, Zhang X, Cheng Z, Chen L, Ding X, Du J, Cai Y, Luo Q, Shen J, Wang Y, Yu L. Toxoplasma gondii inhibits differentiation of C17.2 neural stem cells through Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun. 2016;473:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Zhang X, Su R, Cheng Z, Zhu W, Li Y, Wang Y, Du J, Cai Y, Luo Q, Shen J, Yu L. A mechanistic study of Toxoplasma gondii ROP18 inhibiting differentiation of C17.2 neural stem cells. Parasit Vectors. 2017;10:585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Centers for Disease Control and Prevention. Malaria. Available from: https://www.cdc.gov/malaria/about/biology/index.html. |
| 42. | Thakur RS, Tousif S, Awasthi V, Sanyal A, Atul PK, Punia P, Das J. Mesenchymal stem cells play an important role in host protective immune responses against malaria by modulating regulatory T cells. Eur J Immunol. 2013;43:2070-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Pathak VA, Ghosh K. Erythropoiesis in Malaria Infections and Factors Modifying the Erythropoietic Response. Anemia. 2016;2016:9310905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Silverman PH, Schooley JC, Mahlmann LJ. Murine malaria decreases hemopoietic stem cells. Blood. 1987;69:408-413. [PubMed] |
| 45. | Maggio-Price L, Brookoff D, Weiss L. Changes in hematopoietic stem cells in bone marrow of mice with plasmodium berghei malaria. Blood. 1985;66:1080-1085. [PubMed] |
| 46. | Lopes CS, Daifalla N, Das B, Dias da Silva V, Campos-Neto A. CD271+ Mesenchymal Stem Cells as a Possible Infectious Niche for Leishmania infantum. PLoS One. 2016;11:e0162927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Allahverdiyev AM, Bagirova M, Elcicek S, Koc RC, Baydar SY, Findikli N, Oztel ON. Adipose tissue-derived mesenchymal stem cells as a new host cell in latent leishmaniasis. Am J Trop Med Hyg. 2011;85:535-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Bogdan C, Donhauser N, Döring R, Röllinghoff M, Diefenbach A, Rittig MG. Fibroblasts as host cells in latent leishmaniosis. J Exp Med. 2000;191:2121-2130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 154] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 49. | Gangneux JP, Lemenand O, Reinhard Y, Guiguen C, Guguen-Guillouzo C, Gripon P. In vitro and ex vivo permissivity of hepatocytes for Leishmania donovani. J Eukaryot Microbiol. 2005;52:489-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Dameshghi S, Zavaran-Hosseini A, Soudi S, Shirazi FJ, Nojehdehi S, Hashemi SM. Mesenchymal stem cells alter macrophage immune responses to Leishmania major infection in both susceptible and resistance mice. Immunol Lett. 2016;170:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Cotterell SE, Engwerda CR, Kaye PM. Enhanced hematopoietic activity accompanies parasite expansion in the spleen and bone marrow of mice infected with Leishmania donovani. Infect Immun. 2000;68:1840-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Nguyen Hoang AT, Liu H, Juaréz J, Aziz N, Kaye PM, Svensson M. Stromal cell-derived CXCL12 and CCL8 cooperate to support increased development of regulatory dendritic cells following Leishmania infection. J Immunol. 2010;185:2360-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Planelles L, Thomas M, Pulgar M, Marañón C, Grabbe S, López MC. Trypanosoma cruzi heat-shock protein-70 kDa,alone or fused to the parasite KMP11 antigen, induces functional maturation of murine dendritic cells. Immunol Cell Biol. 2002;80:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Abidin BM, Hammami A, Stäger S, Heinonen KM. Infection-adapted emergency haematopoiesis promotes visceral leishmaniasis. PLoS Pathog. 2017;13:e1006422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 55. | Pinto AI, Brown N, Preham O, Doehl JSP, Ashwin H, Kaye PM. TNF signalling drives expansion of bone marrow CD4+ T cells responsible for HSC exhaustion in experimental visceral leishmaniasis. PLoS Pathog. 2017;13:e1006465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Mahla RS. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int J Cell Biol. 2016;2016:6940283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 342] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 57. | World Health Organization. Chagas disease (American trypanosomiasis). Available from: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis). |
| 58. | Malik LH, Singh GD, Amsterdam EA. The Epidemiology, Clinical Manifestations, and Management of Chagas Heart Disease. Clin Cardiol. 2015;38:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 59. | Bocchi EA, Bestetti RB, Scanavacca MI, Cunha Neto E, Issa VS. Chronic Chagas Heart Disease Management: From Etiology to Cardiomyopathy Treatment. J Am Coll Cardiol. 2017;70:1510-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 60. | Goldenberg RC, Jelicks LA, Fortes FS, Weiss LM, Rocha LL, Zhao D, Carvalho AC, Spray DC, Tanowitz HB. Bone marrow cell therapy ameliorates and reverses chagasic cardiomyopathy in a mouse model. J Infect Dis. 2008;197:544-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Soares MB, Lima RS, Rocha LL, Takyia CM, Pontes-de-Carvalho L, de Carvalho AC, Ribeiro-dos-Santos R. Transplanted bone marrow cells repair heart tissue and reduce myocarditis in chronic chagasic mice. Am J Pathol. 2004;164:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 62. | Soares MB, Lima RS, Souza BS, Vasconcelos JF, Rocha LL, Dos Santos RR, Iacobas S, Goldenberg RC, Lisanti MP, Iacobas DA, Tanowitz HB, Spray DC, Campos de Carvalho AC. Reversion of gene expression alterations in hearts of mice with chronic chagasic cardiomyopathy after transplantation of bone marrow cells. Cell Cycle. 2011;10:1448-1455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Carvalho AB, Goldenberg RCDS, Campos de Carvalho AC. Cell therapies for Chagas disease. Cytotherapy. 2017;19:1339-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Vilas-Boas F, Feitosa GS, Soares MB, Pinho-Filho JA, Mota A, Almeida AJ, Carvalho C, de Carvalho HG, de Oliveira AD, dos Santos RR. Bone marrow cell transplantation to the myocardium of a patient with heart failure due to chagas' disease. Arq Bras Cardiol. 2004;82:185-187, 181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Ribeiro Dos Santos R, Rassi S, Feitosa G, Grecco OT, Rassi A, da Cunha AB, de Carvalho VB, Guarita-Souza LC, de Oliveira W, Tura BR, Soares MB, Campos de Carvalho AC; Chagas Arm of the MiHeart Study Investigators. Cell therapy in Chagas cardiomyopathy (Chagas arm of the multicenter randomized trial of cell therapy in cardiopathies study): a multicenter randomized trial. Circulation. 2012;125:2454-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 66. | Jasmin, Jelicks LA, Koba W, Tanowitz HB, Mendez-Otero R, Campos de Carvalho AC, Spray DC. Mesenchymal bone marrow cell therapy in a mouse model of chagas disease. Where do the cells go? PLoS Negl Trop Dis. 2012;6:e1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Jasmin, Jelicks LA, Tanowitz HB, Peters VM, Mendez-Otero R, de Carvalho ACC, Spray DC. Molecular imaging, biodistribution and efficacy of mesenchymal bone marrow cell therapy in a mouse model of Chagas disease. Microbes Infect. 2014;16:923-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Larocca TF, Souza BS, Silva CA, Kaneto CM, Alcantara AC, Azevedo CM, Castro MF, Macambira SG, Soares MB, Ribeiro-dos-Santos R. Transplantation of adipose tissue mesenchymal stem cells in experimental chronic chagasic cardiopathy. Arq Bras Cardiol. 2013;100:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | World Health Organization. Leishmaniasis. Available from: http://www.who.int/mediacentre/factsheets/fs375/en/. |
| 70. | Bifari F, Lisi V, Mimiola E, Pasini A, Krampera M. Immune Modulation by Mesenchymal Stem Cells. Transfus Med Hemother. 2008;35:194-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Pereira JC, Ramos TD, Silva JD, de Mello MF, Pratti JES, da Fonseca-Martins AM, Firmino-Cruz L, Kitoko JZ, Chaves SP, Gomes DCO, Diaz BL, Rocco PRM, de Matos Guedes HL. Effects of Bone Marrow Mesenchymal Stromal Cell Therapy in Experimental Cutaneous Leishmaniasis in BALB/c Mice Induced by Leishmania amazonensis. Front Immunol. 2017;8:893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Schwarz T, Remer KA, Nahrendorf W, Masic A, Siewe L, Müller W, Roers A, Moll H. T cell-derived IL-10 determines leishmaniasis disease outcome and is suppressed by a dendritic cell based vaccine. PLoS Pathog. 2013;9:e1003476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Olveda DU, Li Y, Olveda RM, Lam AK, Chau TN, Harn DA, Williams GM, Gray DJ, Ross AG. Bilharzia: Pathology, Diagnosis, Management and Control. Trop Med Surg. 2013;1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 74. | Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1526] [Cited by in RCA: 1526] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 75. | Gray DJ, Ross AG, Li YS, McManus DP. Diagnosis and management of schistosomiasis. BMJ. 2011;342:d2651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 266] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 76. | Fikry H, Gawad SA, Baher W. Therapeutic Potential of Bone Marrow-Derived Mesenchymal Stem Cells on Experimental Liver Injury Induced by Schistosoma mansoni: A Histological Study. Int J Stem Cells. 2016;9:96-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (2)] |
| 77. | El-Shennawy SF, Abdel Aaty HE, Radwan NA, Abdel-Hameed DM, Alam-Eldin YH, El-Ashkar AM, Abu-Zahra FA. Therapeutic Potential of Mesenchymal Stem Cells on Early and Late Experimental Hepatic Schistosomiasis Model. J Parasitol. 2015;101:587-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 564] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 79. | Xu H, Qian H, Zhu W, Zhang X, Yan Y, Mao F, Wang M, Xu H, Xu W. Mesenchymal stem cells relieve fibrosis of Schistosoma japonicum-induced mouse liver injury. Exp Biol Med (Maywood). 2012;237:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Elkhafif N, El Baz H, Hammam O, Hassan S, Salah F, Mansour W, Mansy S, Yehia H, Zaki A, Magdy R. CD133(+) human umbilical cord blood stem cells enhance angiogenesis in experimental chronic hepatic fibrosis. APMIS. 2011;119:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | World Health Organization. Malaria. Available from: https://www.who.int/news-room/fact-sheets/detail/malaria. |
| 82. | Basu S, Sahi PK. Malaria: An Update. Indian J Pediatr. 2017;84:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 83. | Belyaev NN, Brown DE, Diaz AI, Rae A, Jarra W, Thompson J, Langhorne J, Potocnik AJ. Induction of an IL7-R(+)c-Kit(hi) myelolymphoid progenitor critically dependent on IFN-gamma signaling during acute malaria. Nat Immunol. 2010;11:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 84. | Hansen DS, Schofield L. Natural regulatory T cells in malaria: host or parasite allies? PLoS Pathog. 2010;6:e1000771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 85. | Wang W, Qian H, Cao J. Stem cell therapy: a novel treatment option for cerebral malaria? Stem Cell Res Ther. 2015;6:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Souza MC, Silva JD, Pádua TA, Torres ND, Antunes MA, Xisto DG, Abreu TP, Capelozzi VL, Morales MM, Sá Pinheiro AA, Caruso-Neves C, Henriques MG, Rocco PR. Mesenchymal stromal cell therapy attenuated lung and kidney injury but not brain damage in experimental cerebral malaria. Stem Cell Res Ther. 2015;6:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 87. | Greenwood HL, Thorsteinsdottir H, Perry G, Renihan J, Singer P, Daar A. Regenerative medicine: New opportunities for developing countries. International Journal of Biotechnology. 2006;3:60-77. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 88. | Food and Drug Administration. Approved cellular and gene therapy products. Available from: https://www.fda.gov/biologicsbloodvaccines/cellulargenetherapyproducts/approvedproducts/default.htm. |
| 89. | Service NB. Guidelines for the blood transfusion services in the united kingdom. Norwich, UK: The Stationary Office, 2013. |
| 90. | Moradin N, Descoteaux A. Leishmania promastigotes: building a safe niche within macrophages. Front Cell Infect Microbiol. 2012;2:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 91. | Rittig MG, Bogdan C. Leishmania-host-cell interaction: Complexities and alternative views. Parasitol Today. 2000;16:292-297. [PubMed] |
| 92. | Komitopoulou A, Tzenou T, Baltadakis J, Apostolidis J, Karakasis D, Harhalakis N. Is leishmaniasis an "unusual suspect" of infection in allogeneic transplantation? Transpl Infect Dis. 2014;16:1012-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 93. | Dantas Brito M, Campilho F, Branca R, Pinho Vaz C, Silva C, Sousa T, Mendes C, Campos A. Visceral leishmaniasis: a differential diagnosis to remember after bone marrow transplantation. Case Rep Hematol. 2014;2014:587912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 94. | Tatura SNN, Gunawan S, Bernadus J, Sandjoto S. Plasmodium falciparum found in the bone marrow of a child in Manado City, East Indonesia: A case report. Asian Pac J Trop Med. 2017;10:1015-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 95. | Skoglund CR. Vasodilatation in human skin induced by low-amplitude high-frequency vibration. Clin Physiol. 1989;9:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Martín-Dávila P, Norman F, Fortún-Abete J, Píris M, Lovatti R, Rubio JM, Martinez-Pérez A, Graus J, Ta G, Villarubia J, Mahillo B, López-Vélez R. Donor-derived multiorgan transmission of mixed P. malariae and P. ovale infection: Impact of globalization on post-transplant infections. Transpl Infect Dis. 2018;20:e12938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Ladeb S, Ben Abdejlil N, Fakhfakh N, Lakhal A, Belloumi D, Ben Hamed L, Kallel A, Torjman L, El Fatimi R, Hmida S, Kallel K, Ben Othman T. Plasmodium falciparum infection transmitted by transfusion: A cause of hemophagocytic syndrome after bone marrow tranplantation in a non-endemic country. Transpl Infect Dis. 2018;20:e12887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 98. | Martino R, Maertens J, Bretagne S, Rovira M, Deconinck E, Ullmann AJ, Held T, Cordonnier C. Toxoplasmosis after hematopoietic stem cell transplantation. Clin Infect Dis. 2000;31:1188-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 99. | Florescu DF, Sandkovsky U. Cryptosporidium infection in solid organ transplantation. World J Transplant. 2016;6:460-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 100. | Grosgogeat Y, Bessede P, Himbert J. [Myocardial infarct with fatal development in the young patient. Anatomical study]. Pathol Microbiol (Basel). 1967;30:727-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Carvalho-Gontijo R, Moreira DR, Resende M, Costa-Silva MF, Peruhype-Magalhães V, Ribeiro CMF, Ribeiro DD, Silvestre R, Cordeiro-da-Silva A, Martins-Filho OA, Teixeira-Carvalho A. Infection of hematopoietic stem cells by Leishmania infantum increases erythropoiesis and alters the phenotypic and functional profiles of progeny. Cell Immunol. 2018;326:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 102. | Passeri E, Jones-Brando L, Bordón C, Sengupta S, Wilson AM, Primerano A, Rapoport JL, Ishizuka K, Kano S, Yolken RH, Sawa A. Infection and characterization of Toxoplasma gondii in human induced neurons from patients with brain disorders and healthy controls. Microbes Infect. 2016;18:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 103. | Tanaka N, Ashour D, Dratz E, Halonen S. Use of human induced pluripotent stem cell-derived neurons as a model for Cerebral Toxoplasmosis. Microbes Infect. 2016;18:496-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 104. | Teranishi H. [Infection of Schwann cells along corneal stromal nerve fiber in experimental herpes simplex keratitis]. Nippon Ganka Gakkai Zasshi. 1974;78:1079-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 1.9] [Reference Citation Analysis (0)] |