Copyright
©The Author(s) 2016.
World J Stem Cells. Aug 26, 2016; 8(8): 260-267
Published online Aug 26, 2016. doi: 10.4252/wjsc.v8.i8.260
Published online Aug 26, 2016. doi: 10.4252/wjsc.v8.i8.260
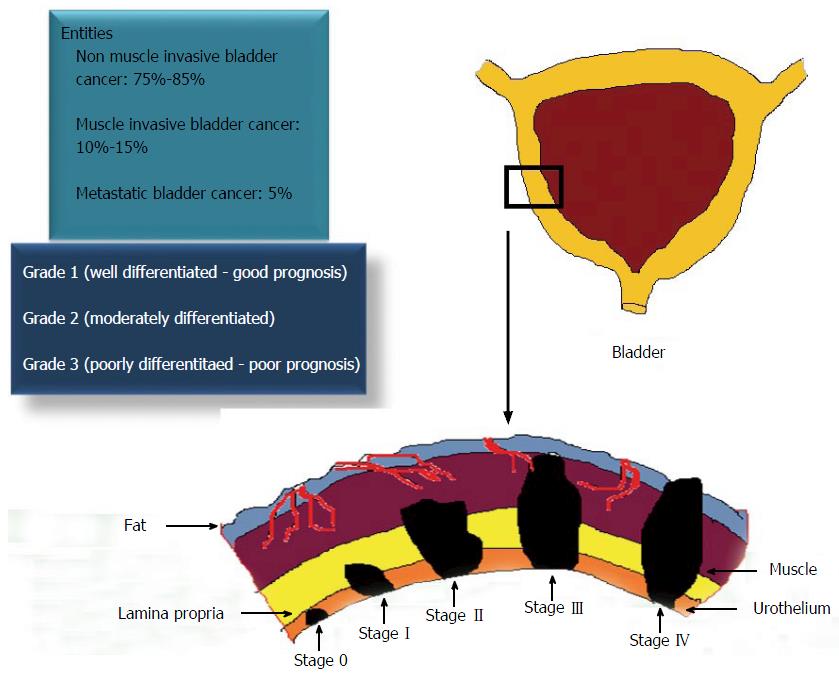
Figure 1 Staging, grading and prognosis of urothelial carcinoma of the bladder.
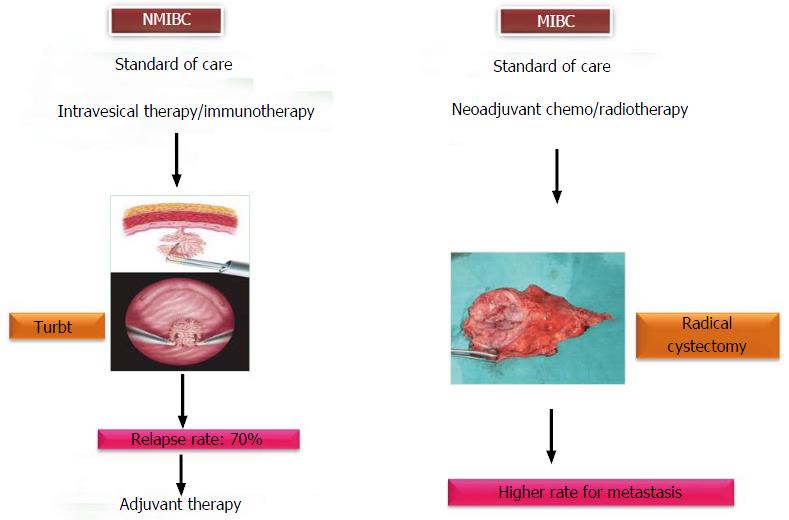
Figure 2 Multimodality approaches for urothelial carcinoma of the bladder.
NMIBC: Non-muscle invasive bladder cancer; MIBC: Muscle invasive bladder cancer.
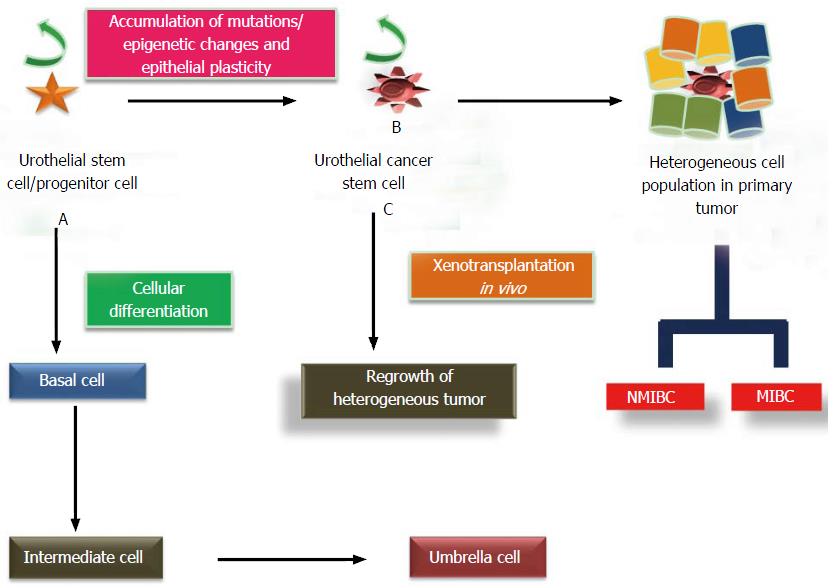
Figure 3 Cellular differentiation and mutational transformation of urothelial stem cells and dual pathways of carcinogenesis.
A: Cellular differentiation of UroSCs (exist in the form of clonal patches in basal layer) gives rise to basal cells which further differentiate to intermediate cells and then into single layer of umbrella cells; B: Mutational insults including epithelial plasticity result in malignant transformation of UroSCs into self-renewing UroCSCs which undergo aberrant activation and differentiation to form papillary non muscle invasive and muscle invasive bladder cancer; C: In vivo xenotransplantation of a small number of UroCSCs that have the potential for the regrowth of heterogeneous tumor cells. UroCSCs: Urothelial cancer stem cells; NMIBC: Non-muscle invasive bladder cancer; MIBC: Muscle invasive bladder cancer.
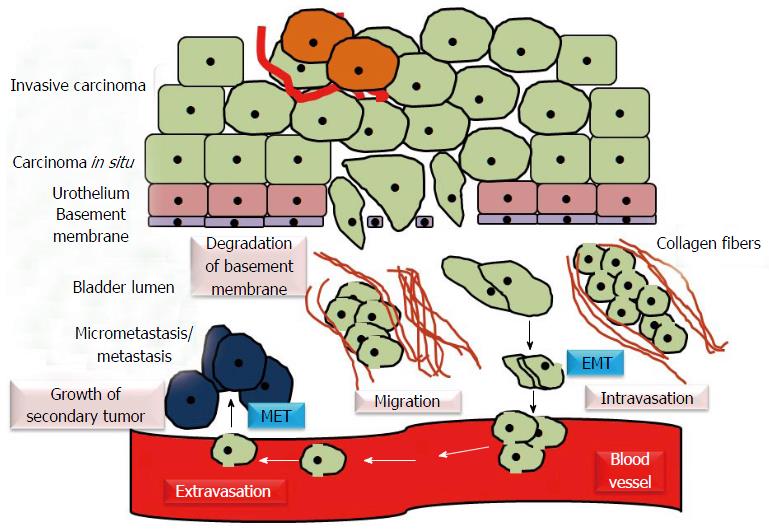
Figure 4 Epithelial mesenchymal transition and mesenchymal epithelial transition in urothelial carcinoma.
EMT/epithelial plasticity allows invasive bladder cancer cells to become motile, invade the surrounding tissues and intravasate. Through bloodstream, primary tumor cells move to distant sites, extravasate, colonize the target organs and establish the metastasis. MET induces regrowth and re-establishment of cancer cells with epithelial phenotype at secondary/metastatic sites. EMT: Epithelial mesenchymal transition; MET: Mesenchymal epithelial transition.
- Citation: Garg M. Epithelial plasticity in urothelial carcinoma: Current advancements and future challenges. World J Stem Cells 2016; 8(8): 260-267
- URL: https://www.wjgnet.com/1948-0210/full/v8/i8/260.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i8.260