Copyright
©The Author(s) 2015.
World J Stem Cells. Jul 26, 2015; 7(6): 922-944
Published online Jul 26, 2015. doi: 10.4252/wjsc.v7.i6.922
Published online Jul 26, 2015. doi: 10.4252/wjsc.v7.i6.922
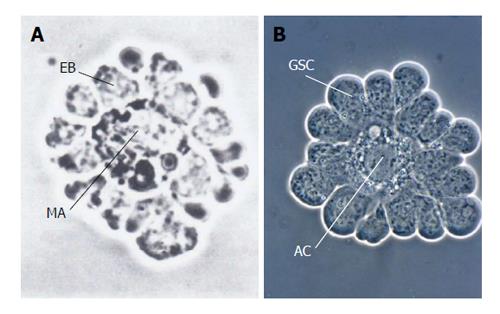
Figure 1 Simple systems of the stem cell/progenitor-niche interaction: isolated erythroblastic island and isolated apical complex from an insect exhibit strong resemblances.
A: Erythroblastic island from dissociated rat bone marrow. A corona of erythroblasts (EB) has extensive cell-cell contact with their niche, the central macrophage (MA) (from Bessis et al[93] with kind permission of Springer Verlag, Berlin, Heidelberg); B: Isolated testicular apical complex from Locusta migratoria. Germline stem cells (GSC) surround the apical cell (AC), which represents the niche (besides the peripheral cyst stem cells, which were removed) (From Dorn et al[64]).
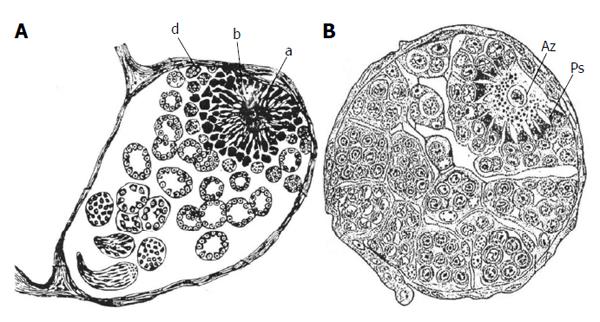
Figure 2 Early histological studies on testicular follicles in butterflies (Lepidoptera) that depict the complex structures of the apical complexes.
A: Testicular follicle of the silkmoth Bombyx mori which includes the apical complex (a, b, d). The limited light microscopical resolution caused some misinterpretation concerning the identity of cell types: the central apical cell (a) was considered to be a “germ cell” (“Keimzelle”) with radial extensions. The germline stem cells were described as clumps of protoplasm with nuclei (b, d) (from Verson[7]); B: Testicular follicle of the cabbage white butterfly Pieris brassicae. The relationship between apical cell (Az) (also called Verson cell) and germline stem cells (Ps) is correctly described: The germline stem cells send projections toward the apical cell and their tips penetrate the apical cell. A layer of cells surround the germline stem cells, the cyst stem cell which, however, were not identified as such (from Zick[8]).
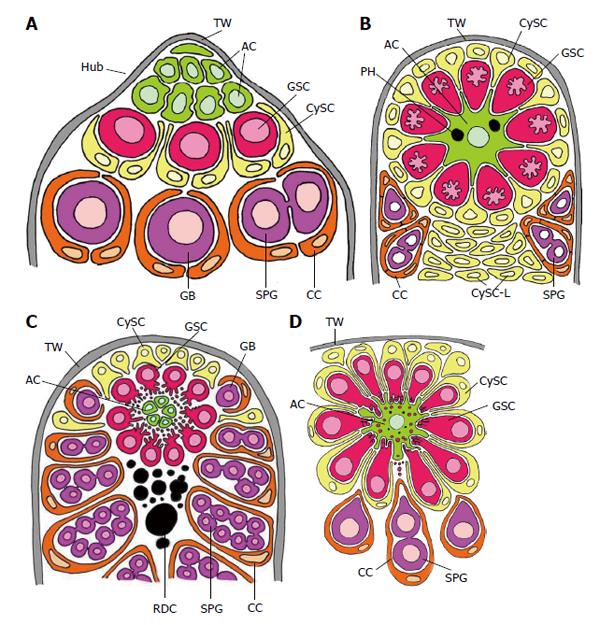
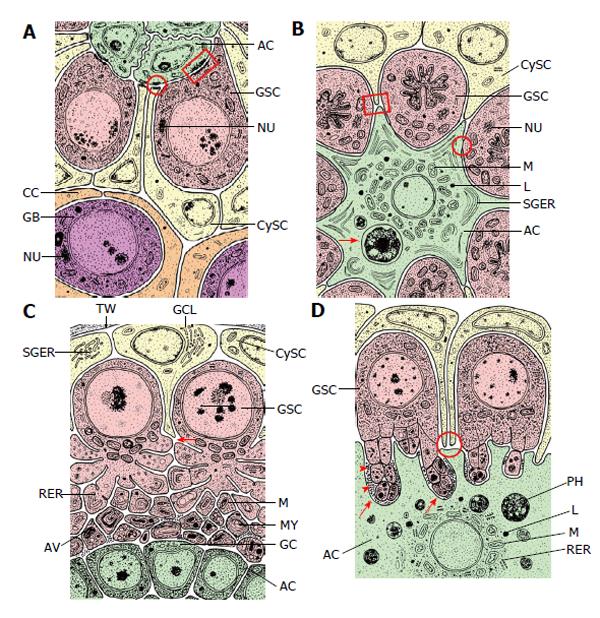
Figure 3 Schematized longitudinal sections through the apices of testicular follicles of four insect species.
The order of the images from (A) to (D) is arranged according to increasing complexity of the structural relationships between germline stem cells (GSCs) and their niche. It should be noted that the order is not in congruence with the positions of the species in the natural insect system based on evolutionary progress. A: Drosophila melanogaster. The ACs (hub cells) are located in a small terminal appendix of the follicle, the hub (HUB), where many of them border the testicular wall (TW). (The testicular wall consists of an outer pigment layer resting on a basal lamina and a muscle cell layer sitting on an inner basal lamina.) The testicular wall does not provide a hemolymph-testis barrier. A spermatogonial is constituted by two CCs which protect the spermatogonia against hemolymph. Both GSCs and CySCs contact ACs (Adapted from Hardy et al[61]); B: Locusta migratoria. Depicted is the rosette-like arrangement of GSCs and CySCs with the single AC in the center. The AC of this example includes two phagocytized GSCs (see above). Besides GSCs also CySCs contact extensions of the star-like AC. A plug of CySC-like cells (CySC-L) is located below the apical rosette. The wall of the spermatogonial cysts is composed of numerous CCs (Adapted from Szöllösi et al[154] and Dorn et al[64]); C: Oncopeltus fasciatus. The cellular extensions of GSCs and segregated vesicles surround the surface of ACs. CySCs cover only the distal part of the apical rosette; they do not make contacts with the ACs. Proximal to the rosette, remnants of degraded GSCs and probably young cysts amass (RDC). Cysts form at the lateral parts of the rosette (Adapted from Schmidt et al[65]); D: Lymantria dispar. Note that the cellular extensions of GSCs protrude into the large singular AC. The extensions autotomize and the segregated vesicles are phagocytized by the AC. Each GSC is affiliated with one CySC. During early larval development the AC is attached to the TW [comparable to the situation in Drosophila, where ACs (i.e., hub cells) are lifelong attached to the TW], but separates with progressing development. The apical complex then adopts a spherical organization. (Adapted from Klein[66]). AC: Apical cell (often called hub cell in Drosophila) (green); CC: Cyst cell (orange); CySC: Cyst stem cell (yellow); GB: Gonialblast (purple); GSC: Germline stem cell (red); SPG: Spermatogonia (purple); TW: Testicular wall (grey).
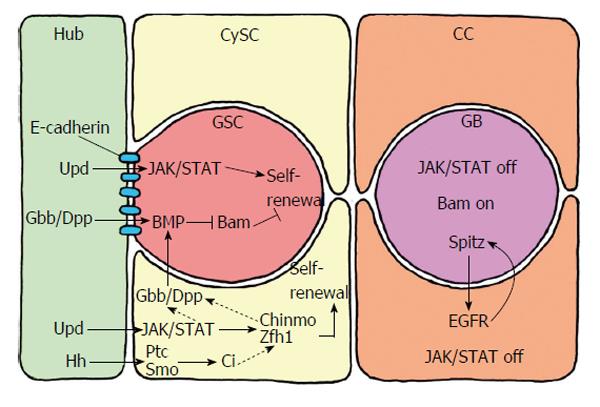
Figure 4 Short range signalling between the germline stem cells and their niche, which consists of the hub cells and the cyst stem cells.
The hub cells secrete the ligand Upd, which activates JAK/STAT signalling in GSCs and CySCs. Whereas JAK/STAT activation is sufficient for CySC maintenance and self-renewal, GSC self-renewal requires additional signals. Hub cells and CySCs secrete the ligands Gbb and Dpp, which activate BMP in GSCs. BMP suppresses the transcription of Bam that inhibits the differentiation of GSCs to gonialblasts. BMP activation in GSCs is also supports by Gbb and Dpp produced by the CySCs. Gbb and Dpp in CySCs are conceivably activated by STAT and/or Zfh1 and Chinmo. Hub cells also secrete the ligand Hh (hedge hog), which supports CySC self-renewal in addition to, but independently of, JAK/STAT activity. Hh binds to the transmembrane receptor PTC (patched) of CySC, which releases Smo (smoothened) from repression. This leads to the activation of the transcription factor Ci (cubitus interruptus), which activates the transcription of target genes that support maintenance and self-renewal of CySCs besides JAK/STAT activity. The Hh signalling pathway in CySCs may also affect Zfh1 and, via Gbb/Dpp, influence BMP signalling in GSCs. Thus, Hh signalling in the testis niche apparently has a dual role. CC: Cyst cell; GSCs: Germline stem cells; CySCs: Cyst stem cells.
Figure 5 Fine structural organization (schematized) of the apical complexes (longitudinal sections) of four insect species.
The order (A) to (D) is arranged according to the order in Figure 3. A: Drosophila melanogaster. ACs are “light” and interdigitate richly between themselves. Their organelle equipment is scarce. GSCs are “dark” due to many free ribosomes and characterized by “spongy bodies” which may represent nuage. Adherens junctions are expressed between ACs and GSCs (marked by a rectangle) and between ACs and CySCs (encircled). CySCs are “light” and include few cell organelles (Adapted from Hardy et al[61]); B: Locusta migratoria. The single apical cell of an apical complex is “light” and harbors a complex organelle equipment: in particular a ring of mitochondria (M) and lysosomes (L) around the nucleus, and stacks of sparsely granulated endoplasmic reticulum (SGER) at the cell periphery. Arrow marks a phagocytized and partly lysed GSC. ACs and GSCs are connected by gap-like junctions (encircled). The “dark” GSCs include extremely irregularly shaped nuclei and nuage-like material (NU). Extensions of the “light” CySCs reach to extensions of the star-shaped AC (marked by square) (Adapted from Dorn et al[64]); C: Oncopeltus fasciatus. The ACs are rather small and very “dark”. Cell organelles are inconspicuous besides Golgi complexes (GC) that face bordering GSC vesicles. The “dark” GSCs form cytoplasmic projections toward the ACs that undergo progressing autotomy. In the course of vesicle segregation rough endoplasmic reticulum (RER) and mitochondria (M) increase. Later autophagic vacuoles (AV) and myelin-like bodies (MY) are formed. Arrow points to a presumably newly sprouting cell projection. The “light” CySCs are characterized by extensive Golgi complex-like structures (GCL) that bare often associated with sparsely granulated endoplasmic reticulum (SGER) (Adapted from Dorn et al[60]); D: Lymantria dispar. The single AC of an apical complex is “light” and exhibits a spherical organization: mitochondria (M), lysosomes (L) and rough endoplasmic reticulum (RER) surround the nucleus: The cell periphery shows deep indentations caused by invading, autotomizing GSC projections (arrows). Segregated GSC vesicles are taken up by the AC, and phagosomes (PH) accumulate in the cytoplasm. The “dense” GSCs exhibit projection formation and projection autotomy that closely resembles that of Oncopeltus (see Figure 4C). GSC projections that indent the AC are often surrounded by extracellular granules (arrow heads). CySCs are “light”, and thin extensions reach the surface of the GSCs (encircled) (Adapted from Klein[66]). AC: Apical cell (green); CC: Cyst cell (orange); CySC: Cyst stem cell (yellow); GB: Gonialblast (purple); GSC: Germline stem cell (red); TW: Testicular wall (grey); AV: Autophagic vacuole; GC: Golgi complex; GCL: Golgi complex-like stricture; L: Lysosomes; M: Mitochondrion; MY: Myelin-like body; NU: Nuage; PH: Phagosomes; RER: Rough endoplasmic reticulum; SGER: Sparsely granulated endoplasmic reticulum.
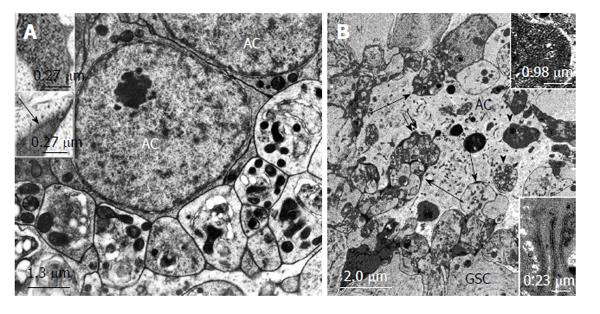
Figure 6 Structural relationships between apical cells and projections and autotomized vesicles of germline stem cells in Oncopeltus fasciatus and Lymantria dispar.
A: Electron micrographs. Oncopeltus fasciatus. Vesicles that are segregated from GSC projections accumulate at the surface of ACs. The vesicles show signs of degeneration. Mitochondria are abundant and appear electron dense. (From Schmidt et al[65]). Upper inset: Intracellular granules in autotomizing GSC projections. Lower inset: Extracellular granules (arrow) between autotomizing GSC projections; B: Electron micrograph. Lymantria dispar. Numerous autotomized GSC projections protrude deeply into the AC (long arrows). Some of the segregated vesicles were apparently taken up by the AC and are being digested (arrow heads). Double arrow points to extracellular granules between GSC vesicles and the AC. Upper inset: Extracellular granules at higher magnification. Lower inset: Tubular indentations into the AC include electron dense material. (From Klein[66]). GSCs: Germline stem cells; AC: Apical cell.
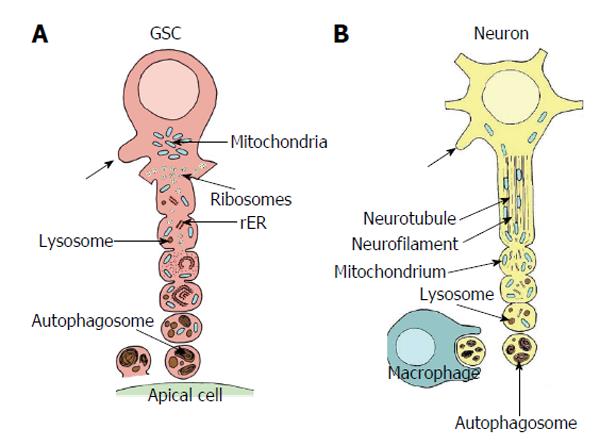
Figure 7 Comparison of autotomizing germline stem cell projection with autodestruction of an injured axon.
A: Sequence of vesicle formation of a GSC projection in Oncopeltus fasciatus. The area of projection formation is characterized by an accumulation of mitochondria (blue). At the base of the projection ribosomes form small clusters (yellow), mitochondria are infrequent. Rough endoplasmic reticulum (rER, orange) and lysosomes (brown) are scarce. With progressing vesicle segregation mitochondria become more frequent and swollen, ribosomes form no longer clusters, rER becomes more prominent and lysosomal bodies increase. Segregated vesicles show many autophagosomes and myelin bodies. They accumulate at the surface of the apical cells (green) and disintegrate. Arrows point to newly sprouting projections (adapted from Dorn et al[60]); B: Sequence of progressive axonal fragmentation after injury. First neurotubules and neurofilaments break down. Then mitochondria accumulate and lysosomes (brown) become more abundant. Finally vesicles which mainly includ autophagosomes and myelin bodies are segregated and taken up by macrophages. Arrows points to newly sprouting axon (adapted from Lingor et al[155]; Beirowski et al[156]; Kerschensteiner et al[157]). GSC: Germline stem cell.
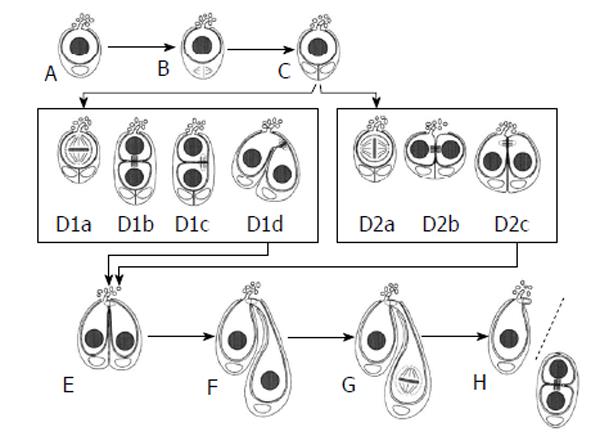
Figure 8 Symmetric and asymmetric germline stem cell division in Lymantria dispar.
Both symmetric and asymmetric GSC divisions occur in this insect species. Both are preceded by the division of the single cyst stem cell that is associated with a GSC (A to C). Asymmetric division is depicted in D1a to D1d. One of the daughter cells is oriented toward the AC and still forms projections, whereas the other daughter cell has no contact with the AC and is devoid of projections (D1b). Then this daughter cell “swivels” round toward the AC (D1d) and adopts a similar position as daughter cells have after a symmetric GSC division (D2a to D2c). In each case, the daughter cell that doesn’t form projections becomes the gonialblast (E to H) (from Klein[66]). GSCs: Germline stem cells; AC: Apical cell.
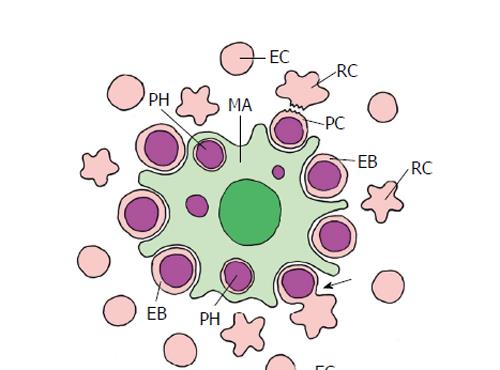
Figure 9 Erythropoiesis in mammal bone marrow (schematized).
The erythroblastic island consists of a central macrophage (MA) that functions as the niche, and the peripheral erythroblasts (EB), that represent stem/progenitor cells. Erythroblasts undergo an enucleation process (arrow) that results in the pyrenocyte (PC) that mainly consists of the erythroblast nucleus, and the nucleus-free reticulocyte (RC). The pyrenocyte is phagocytized by the macrophage (PH, phagosome), whereas the reticulocyte develops to the erythrocyte (EC) (adapted from Chasis et al[4]; Keerthivasan et al[98]).
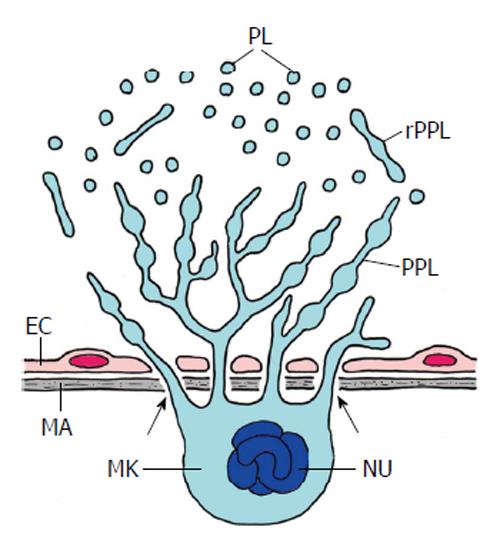
Figure 10 Thrombopoiesis (platelet formation) in mammal bone marrow (schematized).
Platelets are produced by a most spectacular form of cell autotomy performed by the megakaryocyte (MK). Maturation and fragmentation of the megakaryocyte is orchestrated by the vascular niche which consists of the endothelial cells (EC) of sinusoids and the extracellular matrix (MA) of the endothelium. The megakaryocyte is located at the outer surface of the sinusoid epithelium and sends pseudopods through the pores of the endothelium into the lumen of the sinusoids (arrows). The pseudopods branch and form proplatelets (PPL). These are released into the sinusoids (rPPL) followed by the release of platelets (PL). Nu, polyploidy nucleus of the megakaryocyte (Adapted from Patel et al[115]).
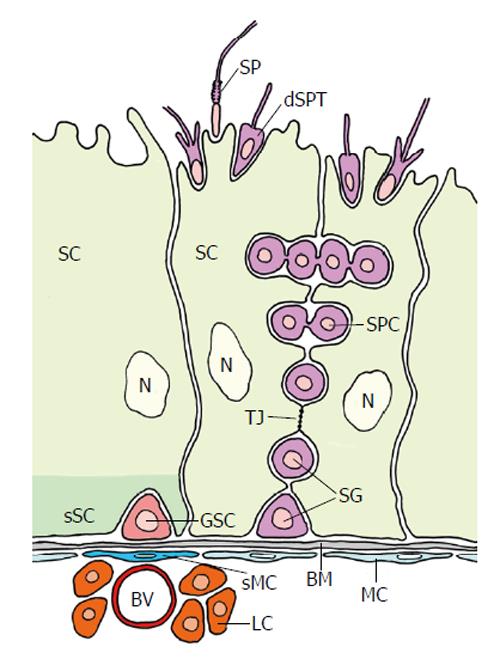
Figure 11 Organization of the mammalian testicular germline stem cell niche (adapted from Yoshida[131]).
The seminiferous tubules exist of large sertoli cells (SC) and spermatocytes (SPC). The putative germline stem cell (GSC) is located at the basement membrane (BM) at an interstitium. It is in close vicinity of a blood vessel (BV) and leydig cells (LC). Myoid cells (MC) that line the BM are thought to be “specialized” MC (sMC) below the GSC. The basal part of the SC that surrounds the GSC presumably performs a specialized “domain” acting as (the main) part of the complex niche. The niche allegedly consists of several additional entities besides the specialized SC domain: the BM, the sMC, the BV, the LC and probably others (see text). Tight junctions (arrow) between SCs establish a blood-testis barrier: GSCs and spermatogonia are exposed to the vascular system, whereas primary spermatocytes (SPC) and the following stages of Spermatogenesis proceed behind the barrier. dSPT: Differentiating spermatid; SP: Spermatozoon; N: Nucleus of SC; Red: GSC; Light green: SC; Dark green: sSC domain surrounding the GSC; Purple: Spermatogonia and different stages of spermatogenesis; Dark blue: sMC; Light blue: MC; Light red: BV; Orange: LC; Grey: BM.
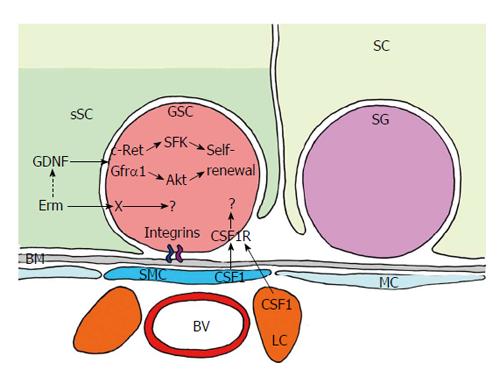
Figure 12 Short range signalling between germline stem cell and its niche.
GDNF is the most important signal produced by the “specialized” basal compartment of SC. Its receptor - c-RET (transmembrane tyrosine kinase receptor) and co-receptor GFRα1, which functions as a glycosylphosphatidylinositol-anchored docking receptor that is needed for GDNF to bind with c-RET - is expressed in GSC, and SFK and Akt pathway are initiated. They control self-renewal and maintenance of GSCs. GSCs also express CSF1R (colony stimulating factor 1 receptor). Its ligand CSF1 is synthesized in “specialized” MCs (sMC) and in leydig cells (LC). Thus, CSF1 may cooperate with GDNF in supporting self-maintenance. Integrins attach the GSCs to the BM and may also contribute to signalling. Red: GSC; Light green: SC; Dark green: sSC domain surrounding the GSC; Purple: Spermatogonium (SG); Dark blue: sMC; Light blue: MC; Light red: BV; Orange: LC; Grey: BM.
- Citation: Dorn DC, Dorn A. Stem cell autotomy and niche interaction in different systems. World J Stem Cells 2015; 7(6): 922-944
- URL: https://www.wjgnet.com/1948-0210/full/v7/i6/922.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i6.922