Copyright
©The Author(s) 2015.
World J Stem Cells. Jul 26, 2015; 7(6): 899-921
Published online Jul 26, 2015. doi: 10.4252/wjsc.v7.i6.899
Published online Jul 26, 2015. doi: 10.4252/wjsc.v7.i6.899
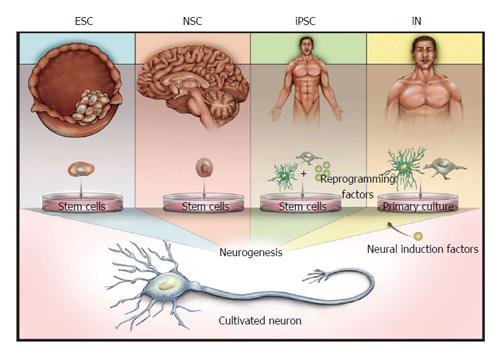
Figure 1 Illustration of the sources of derived neurons.
Embryonic stem cells (ESCs) are derived from the inner cell mass of blastocysts, whereas neural stem cells (NSCs) are derived from several defined niches in the developing or adult brain. Both ESCs and NSCs are capable of neurogenesis without the forced expression of induction factors. Induced pluripotent stem cells (iPSCs) and induced neurons (iNs) can be derived from various tissues, and proceed to neuronal states via either reprogramming to a stem cell phenotype (iPSCs) or direct conversion using neuronal induction factors (iNs).
Figure 2 Neurotypic markers and passive membrane responses are not predictive of functional synaptogenesis.
A: Representative fluorescent immunocytochemistry of i-neurons from several protocols (A1-A4) imaged at 43 d after plating (DAP). SCNs display neurotypic morphologies, including distinct dendrites (Map2) and axons (Tau) around cell nuclei [4’,6-diamidino-2-phenylindole (DAPI)], with distributed and punctate synapsin (top panel) and PSD-95 or gephyrin (bottom panel) often expressed in close proximity to synapsin (insets); B: Although all four i-neuron models displayed intrinsic excitability, including voltage-gated currents, and produced action potentials following current injection, responses were smaller in amplitude and depolarization led to fewer repeated action potentials compared to responses displayed by mouse embryonic stem cell-derived neurons (mESNs, top row); C: Only one of four human i-neuron models displayed synaptic activity on DAP35 or later as indicated by miniature post-synaptic currents in the presence of tetrodotoxin, and those currents were smaller in amplitude, less frequent and slower to return to baseline when compared to mESNs; D: Transmission electron microscopy of single synapses in mESN and i-neuron models (D1) indicates that pre-synaptic compartments of mESNs are densely loaded with vesicles, while i1 is not. This indicates that while the model is capable of producing the morphology, synaptic markers and intrinsic characteristics of synaptogenesis, it lacks the capacity to maintain spontaneous activity and therefore is unable to model physiological synaptic transmission or network activity.
- Citation: Bradford AB, McNutt PM. Importance of being Nernst: Synaptic activity and functional relevance in stem cell-derived neurons. World J Stem Cells 2015; 7(6): 899-921
- URL: https://www.wjgnet.com/1948-0210/full/v7/i6/899.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i6.899