Copyright
©The Author(s) 2015.
World J Stem Cells. Mar 26, 2015; 7(2): 253-265
Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.253
Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.253
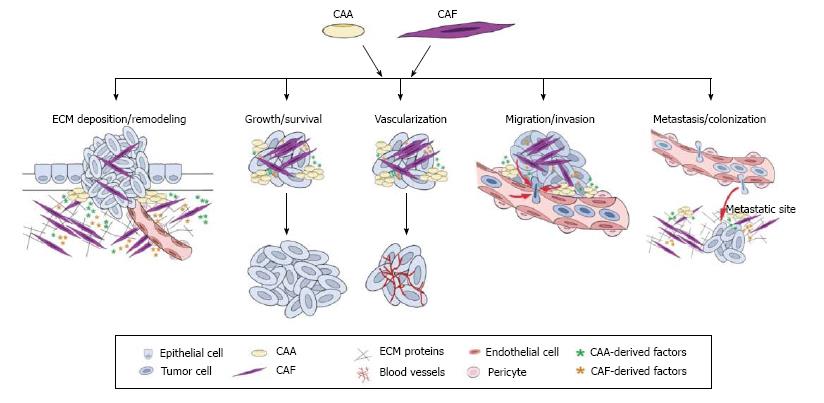
Figure 1 Multifactorial contributions of cancer-associated adipocytes and cancer-associated fibroblasts to tumor progression and metastasis.
Research in the last decade has highlighted the importance of the tumor microenvironment in cancer progression. While there are numerous stromal cell types that contribute to the tumor microenvironment, this illustration depicts roles for cancer-associated adipocytes (CAAs) and cancer-associated fibroblasts (CAFs) in promoting the multiple stages of tumor progression and metastasis. ECM: Extracellular matrix.
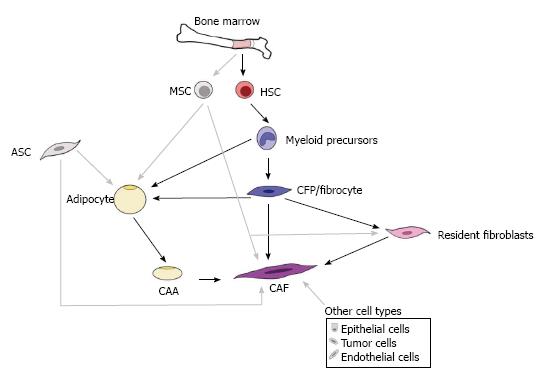
Figure 2 Origins of cancer-associated adipocytes and cancer-associated fibroblasts.
This drawing illustrates the complex and ever-growing understanding of the origins for cancer-associated adipocytes (CAAs) and cancer-associated fibroblasts (CAFs). CAAs and CAFs are generally thought to arise from resident tissue cells, the adipose stem cell (ASC) and resident fibroblast, respectively. However, alternative sources for adipocytes and CAAs have been demonstrated including cells of the bone marrow, specifically those of the myeloid lineage (e.g., macrophages, CFPs, and fibrocytes). In addition to resident fibroblasts, CAFs have been shown to be derived from myeloid progenitors (CFPs, fibrocytes), mesenchymal stromal cells (MSCs), ASCs, CAAs, epithelial cells, tumor cells, and endothelial cells. It is possible that these multiple sources are reflected in the morphological, phenotypic, and functional heterogeneity described for adipocytes and for CAFs. HSC: Hematopoietic stem cell; CFPs: Circulating fibroblast precursors.
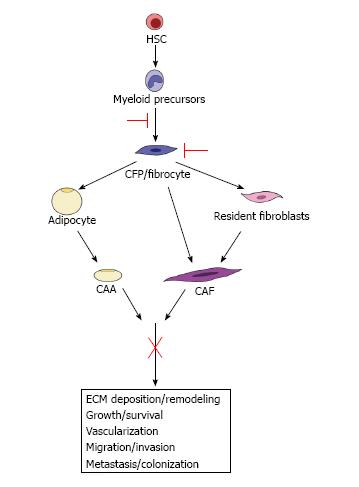
Figure 3 Therapeutic implications.
We hypothesize that the identification of pathways from the hematopoietic stem cell (HSC) to the cancer-associated adipocyte (CAA) and cancer-associated fibroblast (CAF) provides a potential opportunity to target these pro-tumorigenic cells both early in their differentiation and at multiple points in their maturation, which may lead to a more encompassing downstream inhibition of their contributions to tumor progression and metastasis.
- Citation: Xiong Y, McDonald LT, Russell DL, Kelly RR, Wilson KR, Mehrotra M, Soloff AC, LaRue AC. Hematopoietic stem cell-derived adipocytes and fibroblasts in the tumor microenvironment. World J Stem Cells 2015; 7(2): 253-265
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/253.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.253