Copyright
©2014 Baishideng Publishing Group Inc.
World J Stem Cells. Sep 26, 2014; 6(4): 421-431
Published online Sep 26, 2014. doi: 10.4252/wjsc.v6.i4.421
Published online Sep 26, 2014. doi: 10.4252/wjsc.v6.i4.421
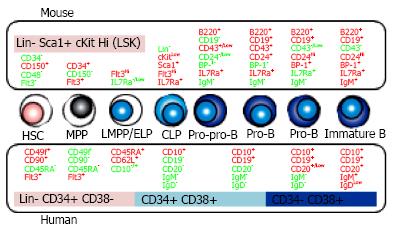
Figure 1 B cell development in bone marrow.
B lineage cells are differentiated from hematopoietic stem cells (HSC) through the several steps defined by the distinct surface phenotypes of positive (red) or negative (green) expression. The comparison between mouse (upper side) and human (lower side) is shown. MPP: Multipotent progenitors; LMPP/ELP: Lymphoid-primed multipotent/ early lymphoid progenitors; CLP: Common lymphoid progenitors; IL7Ra: IL-7 receptor alpha.
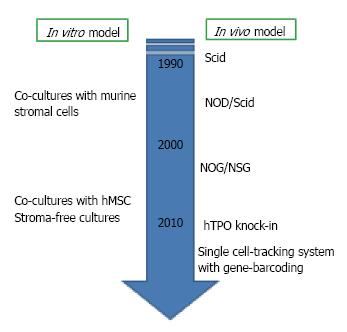
Figure 2 Experimental models for human B lymphopoiesis.
Experimental techniques for studying human B lymphopoiesis have incredibly advanced within these two decades. Now several culture systems with human mesenchymal stem cells (hMSC) or without stromal cells are available. For in vivo studies, the generation of humanized mice has been developed after the discovery of severe combined immune-deficient mouse (Scid). NOG, nonobese diabetic (NOD)-Scid mouse with truncation in the IL-2 receptor common gamma chain; NSG, NOD-Scid mouse with deletion in the IL-2 receptor common gamma chain; hTPO knock-in, RAG-2-/- NSG mouse with humanization of thrombopoietin.
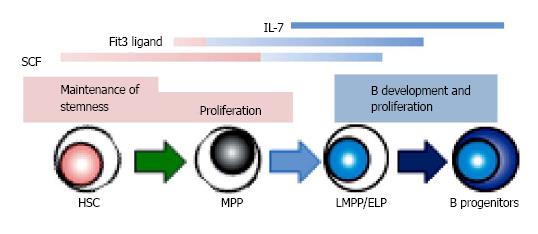
Figure 3 The role of signaling molecules in B lymphopoiesis.
Several cytokines, such as stem cell factor (SCF), Flt3 ligand and IL-7, show the various effects depending on the developmental stages. There are several species differences in the role of cytokines between human and mouse. IL-7 is required for adult mouse B lymphopoiesis, but not for that of human. Recent studies indicate that Flt3 signaling plays a crucial role in lymphoid, but not in HSC or myeloid development in mouse, while human Flt3 ligand affects the survival of hematopoietic stem/ progenitor cells as well as B cell differentiation. HSC: Hematopoietic stem cells; MPP: Multipotent progenitors; LMPP/ELP: Lymphoid-primed multipotent/early lymphoid progenitors.
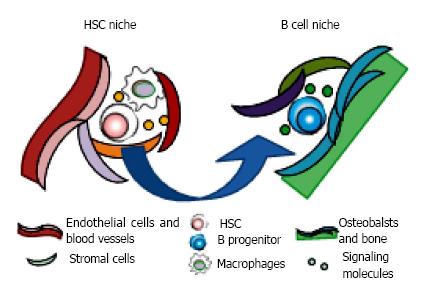
Figure 4 Motility of hematopoietic stem and progenitor cells in bone marrow.
Hematopoietic stem and progenitor cells reside in their own specialized niches where they could preserve the reconstituting and/or differentiating ability. The cellular components and anatomical localization make specialized environments, consisting of soluble and surface-bound signaling molecules, cell-cell contacts, extracellular matrix proteins, and local mechanical environments. The niches for stem cell maintenance and differentiation are distinct. It is believed that hematopoietic stem cells (HSC) self-renew in their niches adjacent to the sinusoidal vasculature with mesenchymal progenitors, endothelial cells, sympathetic neurons, arteries, and macrophages such as osteoclasts. Once the differentiating daughter cell is generated after asymmetrical division of HSC, it moves to the favorable space for undergoing specific lineage commitment. For B lymphopoiesis, progenitors are co-localized with bone-lining oseoblasts in endosteal area.
- Citation: Ichii M, Oritani K, Kanakura Y. Early B lymphocyte development: Similarities and differences in human and mouse. World J Stem Cells 2014; 6(4): 421-431
- URL: https://www.wjgnet.com/1948-0210/full/v6/i4/421.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i4.421