Copyright
©2014 Baishideng Publishing Group Inc.
World J Stem Cells. Jul 26, 2014; 6(3): 322-343
Published online Jul 26, 2014. doi: 10.4252/wjsc.v6.i3.322
Published online Jul 26, 2014. doi: 10.4252/wjsc.v6.i3.322
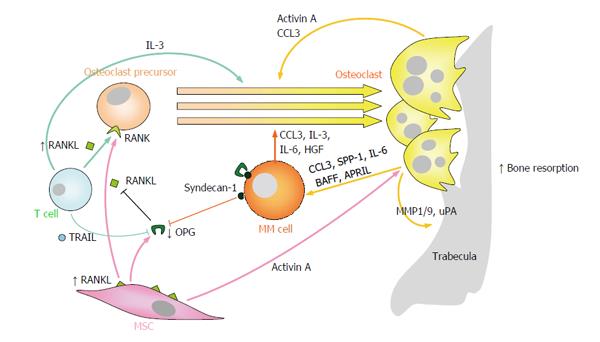
Figure 1 Enhanced osteoclast formation and resorption partially mediates the development of myeloma bone disease.
Numerous “OC-activating factors” produced by multiple myeloma cells and other cells in the bone marrow microenvironment (including RANKL, CCL3, activin A, IL-3, HGF and IL-6) readily promote OC differentiation from OC precursors and/or stimulation of OC resorptive activity. In MM, the RANKL/OPG ratio is clearly favored towards RANKL, both because of increased expression of RANKL on MSCs and T lymphocytes, and because of reduced expression of OPG by MSCs and inactivation of OPG through binding to TRAIL or syndecan-1 on the surfaces of myeloma cells. On the other hand, OCs produce several factors (e.g., IL-6, CCL3, BAFF and APRIL) which promote the growth and survival of multiple myeloma cells. RANKL: Receptor activator of NFκB ligand; NFκB: Nuclear factor-κB; CCL3/MIP1α: Macrophage inflammatory protein 1-α; IL-3/6: Interleukin 3/6; HGF: Hepatocyte growth factor; OPG: Osteoprotegerin; RANK: Receptor activator of NFκB; TRAIL: Tumor necrosis factor-related apoptosis inducing ligand; BAFF: B-cell-activating factor; APRIL: A proliferation-inducing ligand; SPP-1: Osteopontin; MMP1/9: Matrix metalloprotease 1/9; uPA: Urokinase plasminogen activator; MSCs: Mesenchymal stromal cells; OC: Osteoclast; MM: Multiple myeloma.
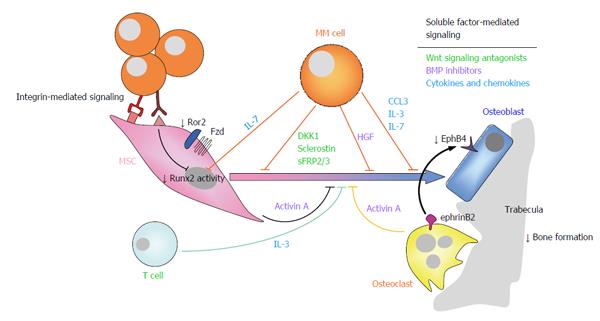
Figure 2 Suppression of osteoblastogenesis and osteoblast function in multiple myeloma is also involved in the pathophysiology of myeloma bone disease.
Myeloma-induced OB suppression is partially mediated by direct cell to cell contact interactions with MSCs, leading to reduced activity of the Runx2/Cbfa1 transcription factor, and to inhibition of non-canonical Wnt5a signaling due to decreased expression of Ror2 in pre-OBs. In addition, soluble factors produced by myeloma cells and cells in the bone marrow microenvironment, such as Wnt signaling antagonists (e.g., DKK1, sclerostin, sFRP-2/3), BMP inhibitors (activin A, TGFβ, HGF), cytokines and chemokines (such as IL-7, TNFα, IL-3, CCL3) and apoptotic factors also contributed to inhibition of osteogenic differentiation and function. Finally, reduced ephrinB2-EphB4 signaling (from OCs to OBs) because of diminished EphB4 expression in MSCs, further contributes to impaired OB differentiation. Ror2: Receptor tyrosine kinase-like orphan receptor 2; DKK1: Dickkopf-1; sFRP-2/3: Secreted frizzled related protein-2/3; TGFβ: Transforming growth factor β; HGF: Hepatocyte growth factor; IL-7/3: Interleukin 7/3; ephrinB2: ephrin-B2 ligand; EphB4: Eph receptor B4; MSCs: Mesenchymal stromal cells; OB: Osteoblast; OC: Osteoclast; CCL3/MIP1α: Macrophage inflammatory protein 1-α; TNFα: Tumor necrosis factor α; BMP: Bone morphogenetic protein; MM: Multiple myeloma.
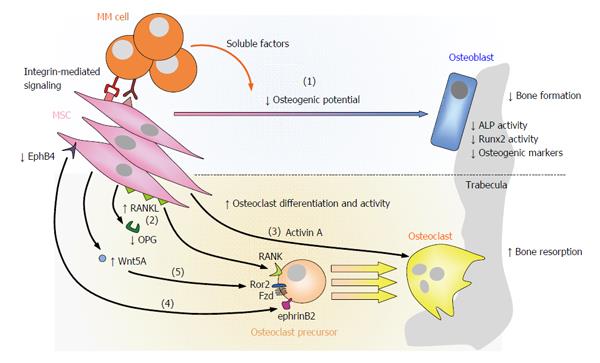
Figure 3 Contribution of mesenchymal stromal cells to myeloma bone disease.
In MM, MSCs contribute to the development of osteolytic lesions not only because of their reduced osteogenic potential [(1)], but also because they promote OC differentiation and hyperactivation at various levels: pMSCs upregulate the expression of RANKL and reduce that of OPG [(2)]; pMSCs augment the secretion of activin A [(3)]; diminished EphB4-ephrinB2 signaling from pMSCs/OBs to OCs allows osteoclastogenesis [(4)]; increased Wnt5a secretion by pMSCs interacting with myeloma cells enhances RANK expression in OC precursors through Ror2, ultimately increasing their sensibility to RANKL [(5)]. RANKL: Receptor activator of NFκB ligand; NFκB: Nuclear factor-κB; OPG: Osteoprotegerin; RANK: Receptor activator of NFκB; EphrinB2: Ephrin-B2 ligand; EphB4: Eph receptor B4; Ror2: Receptor tyrosine kinase-like orphan receptor 2; MSCs: Mesenchymal stromal cells; MM: Multiple myeloma; OC: Osteoclast; OB: Osteoblast; ALP: Alkaline phosphatase; MM: Multiple myeloma.
- Citation: Garcia-Gomez A, Sanchez-Guijo F, del Cañizo MC, San Miguel JF, Garayoa M. Multiple myeloma mesenchymal stromal cells: Contribution to myeloma bone disease and therapeutics. World J Stem Cells 2014; 6(3): 322-343
- URL: https://www.wjgnet.com/1948-0210/full/v6/i3/322.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i3.322