Copyright
©2010 Baishideng.
World J Stem Cells. Jun 26, 2010; 2(3): 39-49
Published online Jun 26, 2010. doi: 10.4252/wjsc.v2.i3.39
Published online Jun 26, 2010. doi: 10.4252/wjsc.v2.i3.39
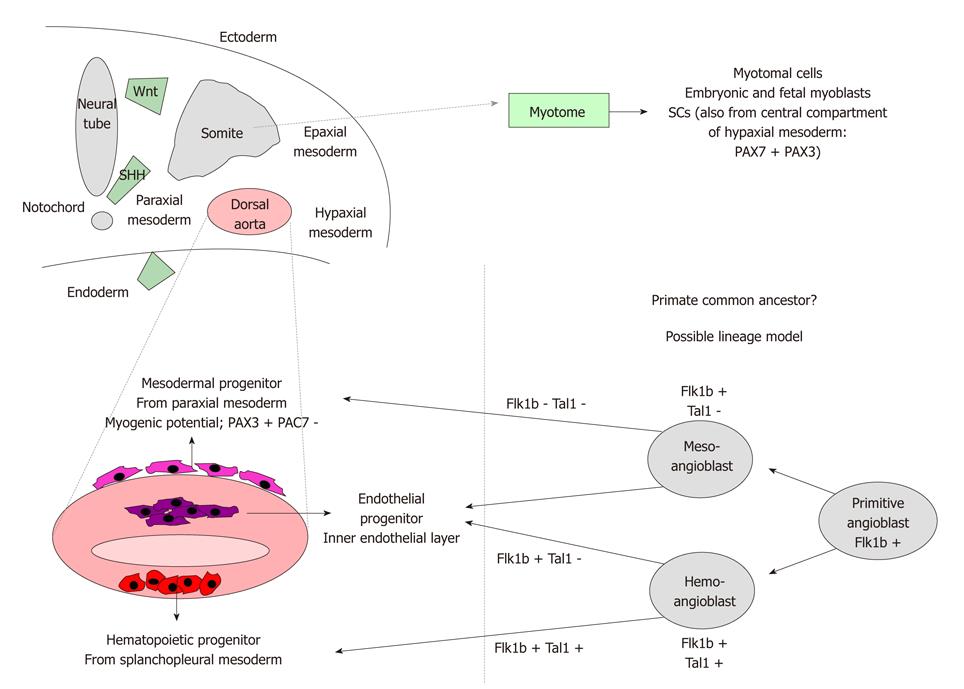
Figure 1 Embryonic origin of muscular progenitors.
Upper panel. Schematic representation of the somitic origin throughout the myotome of classical muscular progenitors-myotomal cells, myoblasts, SCs. A fraction of SCs derives from a population of undifferentiated PAX7+PAX3+ stem cells located in the central compartment of hypaxial mesoderm. Lower panel. Left. Origin of various multipotent progenitors from different areas of dorsal aorta (mesodermal progenitors endowed with the highest myogenic potential, endothelial, hematopoietic progenitors). Wnt, sonic hedgehog and endoderm signalings can influence commitment. Right. Possible lineage model of mesodermal progenitors which could derive from a unique common primitive ancestor (angioblast) (partially developed from Cossu et al[15]). Flk1b: Flit ligand kinase 1b; Tal1: T acute leukemia 1; SHH: Sonic hedgehog.
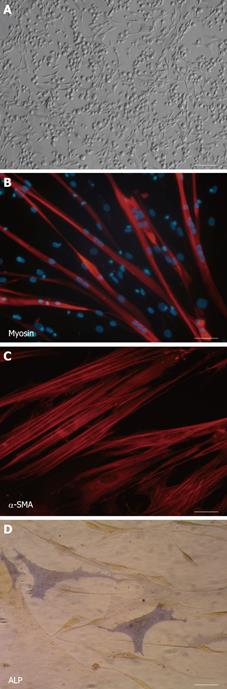
Figure 2 Cell morphology and multilineage differentiation of human mesoangioblasts.
A: Phase contrast image showing human proliferating mesoangioblasts with refractive triangular, adherent and round loosely adherent/floating components. Scale bar: 40 μm; B: Skeletal muscle differentiation. After exposure to normal human myoblasts-conditioned medium and subsequently to differentiation medium, human mesoangioblasts efficiently fuse into myosin-positive fully differentiated myotubes. Scale bar: 40 μm; C: Smooth-muscle differentiation. After treatment with TGFβ human mesoangioblasts stained with anti-α-SMA antibody show approximately 80% positivity. Scale bar: 10 μm; D: Osteoblasts differentiation. After treatment with BMP2 human mesoangioblasts differentiate into strongly ALP-positive osteoblast-like cells. Scale bar: 40 μm.
- Citation: Sancricca C, Mirabella M, Gliubizzi C, Broccolini A, Gidaro T, Morosetti R. Vessel-associated stem cells from skeletal muscle: From biology to future uses in cell therapy. World J Stem Cells 2010; 2(3): 39-49
- URL: https://www.wjgnet.com/1948-0210/full/v2/i3/39.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v2.i3.39