Copyright
©The Author(s) 2025.
World J Stem Cells. May 26, 2025; 17(5): 106547
Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.106547
Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.106547
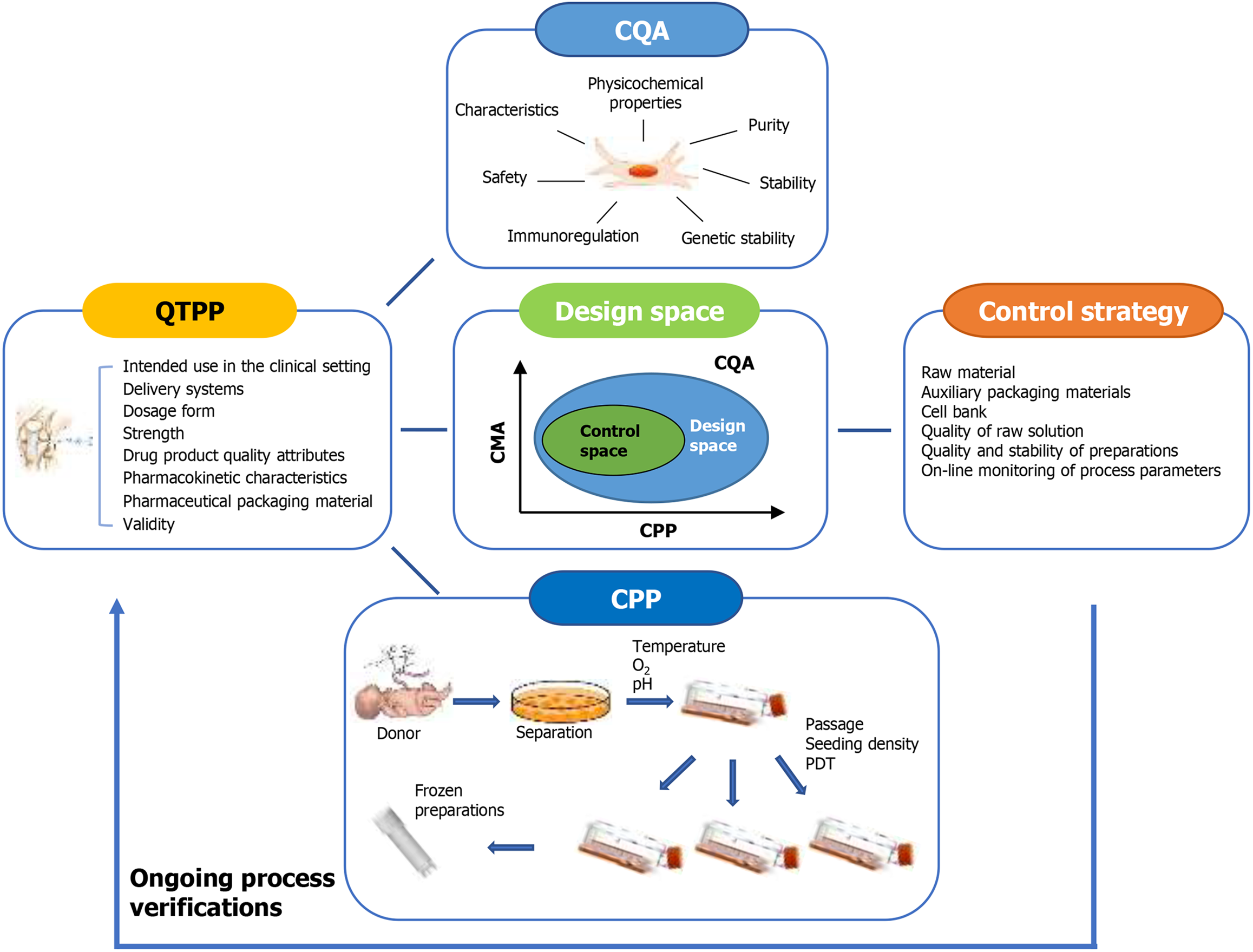
Figure 1 Summary of quality by design process for the production of mesenchymal stem/stromal cells.
CQA: Critical quality attributes; QTPP: Quality Target Product Profile; CMA: Critical material attributes; CPP: Critical process parameters; PDT: Population doubling time.
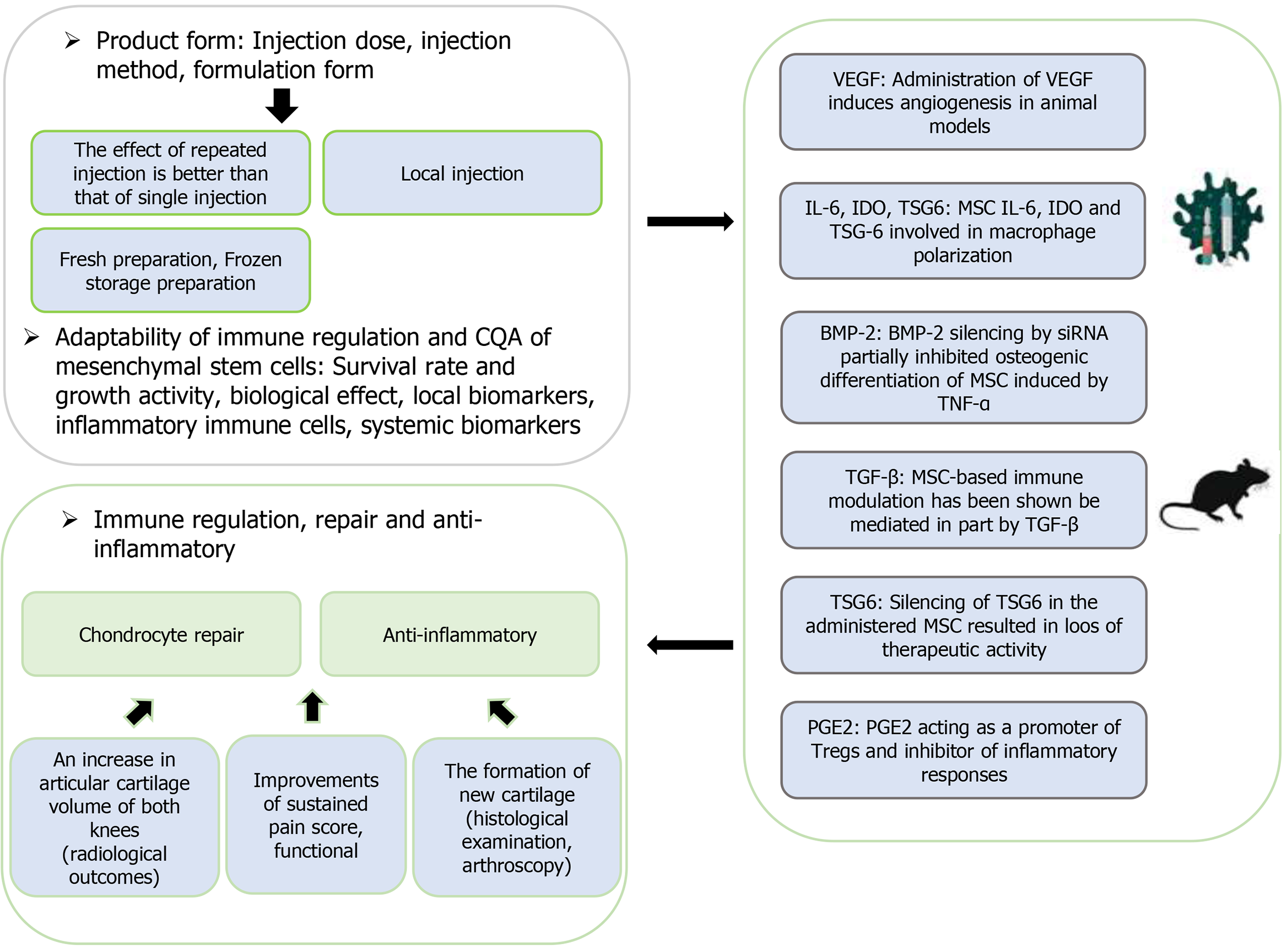
Figure 2 The critical quality attributes-related clinical mechanism of mesenchymal stem/stromal cell-based therapy for knee osteo
- Citation: Yu H, Zhang F, He YC, Zhang LS. Quality by design strategy of human mesenchymal stem/stromal cell drug products for the treatment of knee osteoarthritis. World J Stem Cells 2025; 17(5): 106547
- URL: https://www.wjgnet.com/1948-0210/full/v17/i5/106547.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i5.106547