Copyright
©The Author(s) 2021.
World J Stem Cells. Aug 26, 2021; 13(8): 1151-1159
Published online Aug 26, 2021. doi: 10.4252/wjsc.v13.i8.1151
Published online Aug 26, 2021. doi: 10.4252/wjsc.v13.i8.1151
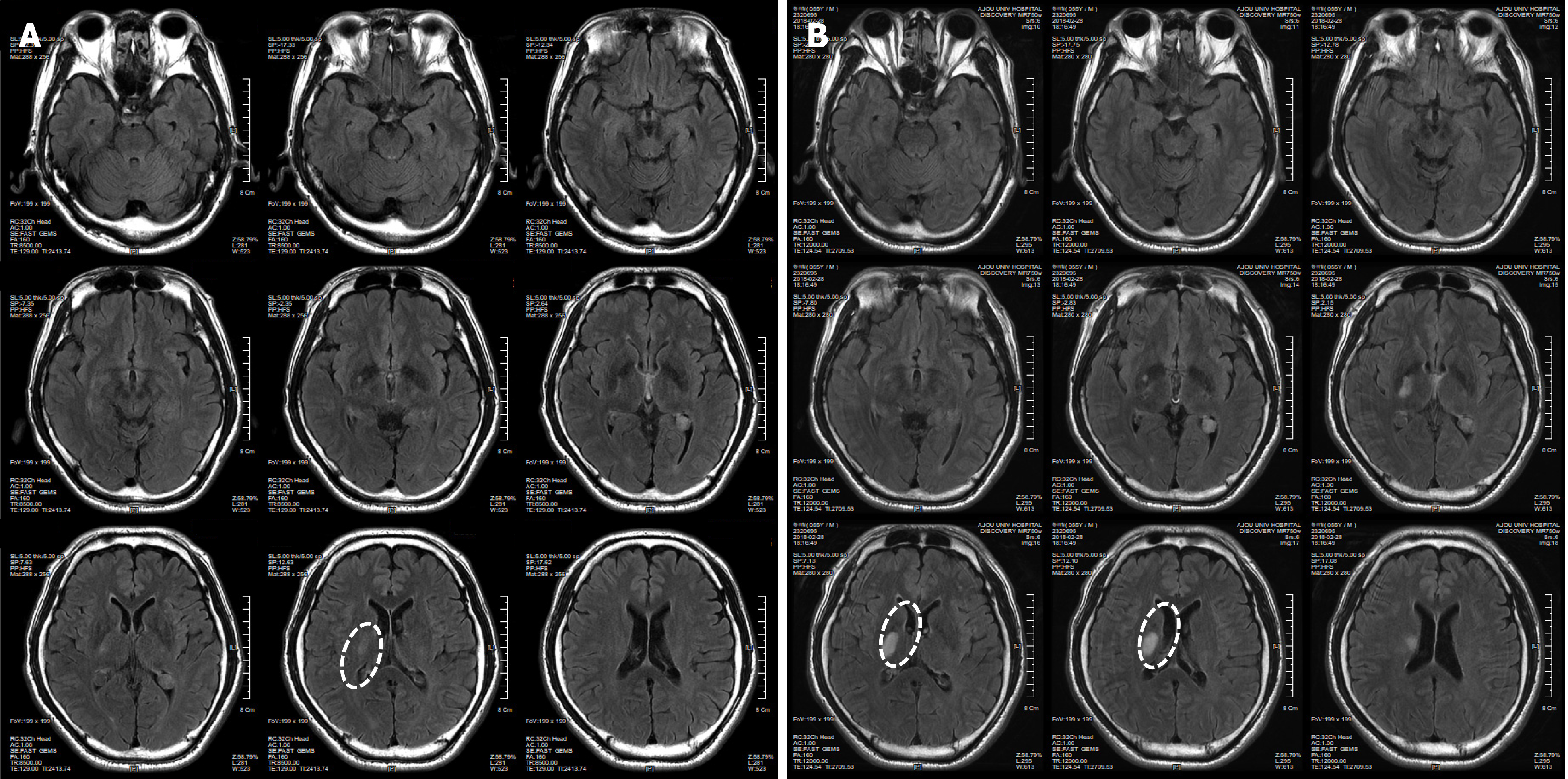
Figure 1 Brain computed tomography images of patient before minimally manipulated human umbilical cord-derived mesenchymal stem cells transplantation.
These are brain computed tomography images of patient at 02/19/2018 (A) and 02/28/2018 (B). The white ovals indicate the lesion site.
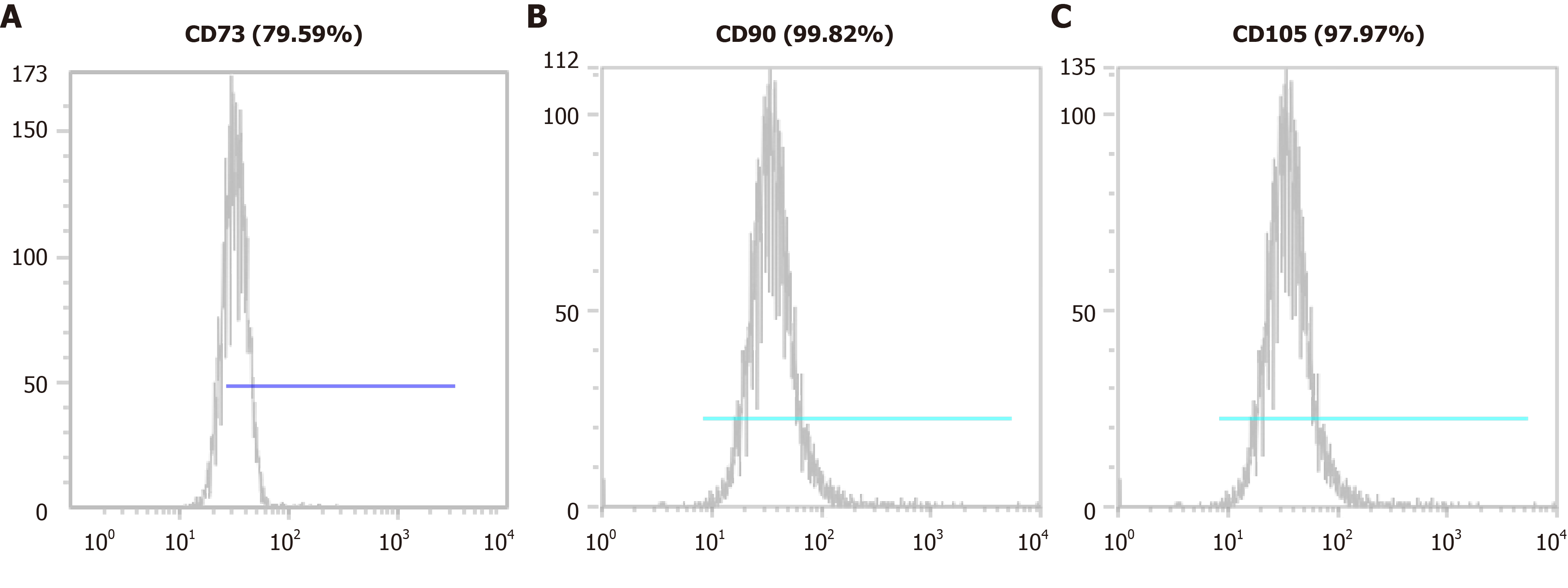
Figure 2 Mesenchymal stem cell marker expression in minimally manipulated umbilical cord-derived mesenchymal stem cells.
The expression marker tested was A: CD73 (79.59%); B: CD90 (99.82%); C: CD105 (97.97%).
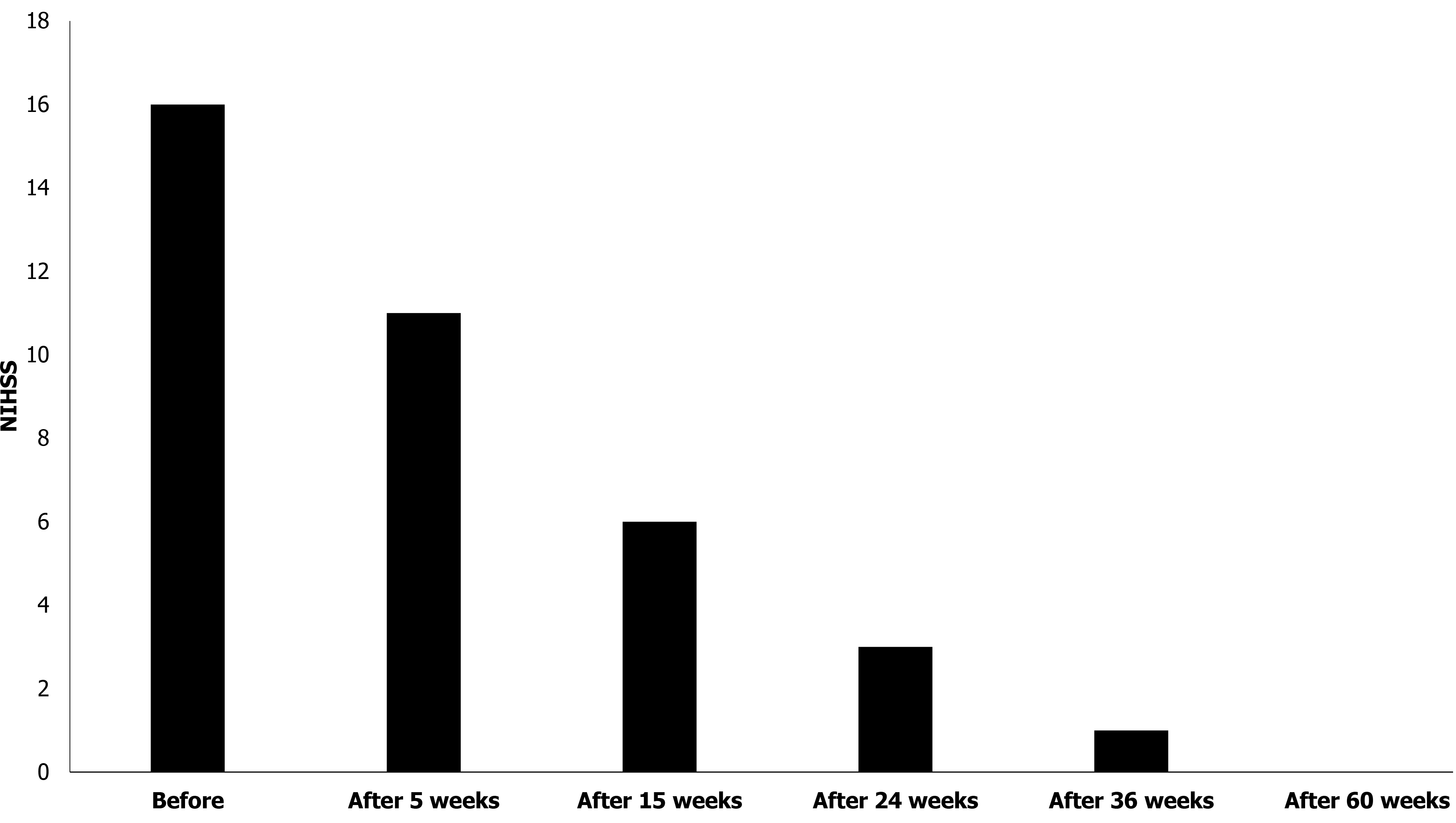
Figure 3 National Institute of Health Stroke Scale score of patient.
The patient’s National Institute of Health Stroke Scale score gradually decreased for 60 wk after the first treatment. NIHSS: National Institute of Health Stroke Scale.
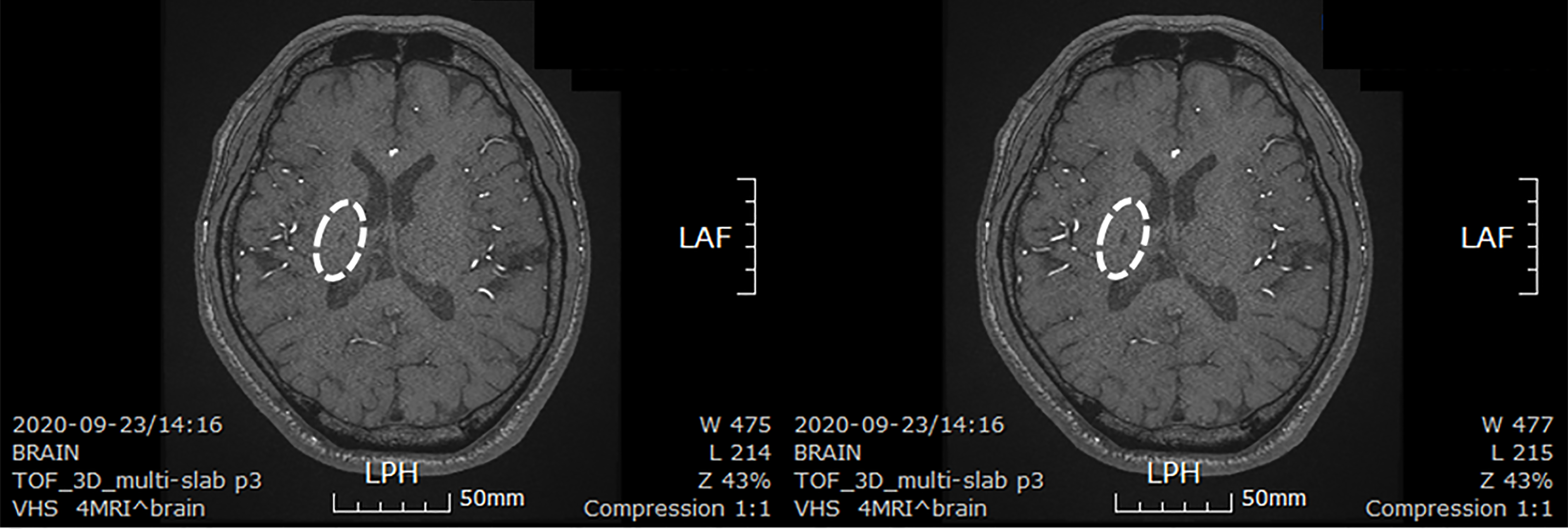
Figure 4 Brain computed tomography images of patient after minimally manipulated human umbilical cord-derived mesenchymal stem cells transplantation.
These are brain computed tomography images of patient at 30 mo after first transplantation. The lesion size decreased from 3 cm × 2 cm to 0.6 cm × 0.3 cm. The white ovals indicate the lesion site. LPH: Left posterior head; LAF: Left anterior frontal.
- Citation: Ahn H, Lee SY, Jung WJ, Lee KH. Treatment of acute ischemic stroke by minimally manipulated umbilical cord-derived mesenchymal stem cells transplantation: A case report . World J Stem Cells 2021; 13(8): 1151-1159
- URL: https://www.wjgnet.com/1948-0210/full/v13/i8/1151.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i8.1151