Copyright
©The Author(s) 2021.
World J Stem Cells. Jul 26, 2021; 13(7): 685-736
Published online Jul 26, 2021. doi: 10.4252/wjsc.v13.i7.685
Published online Jul 26, 2021. doi: 10.4252/wjsc.v13.i7.685
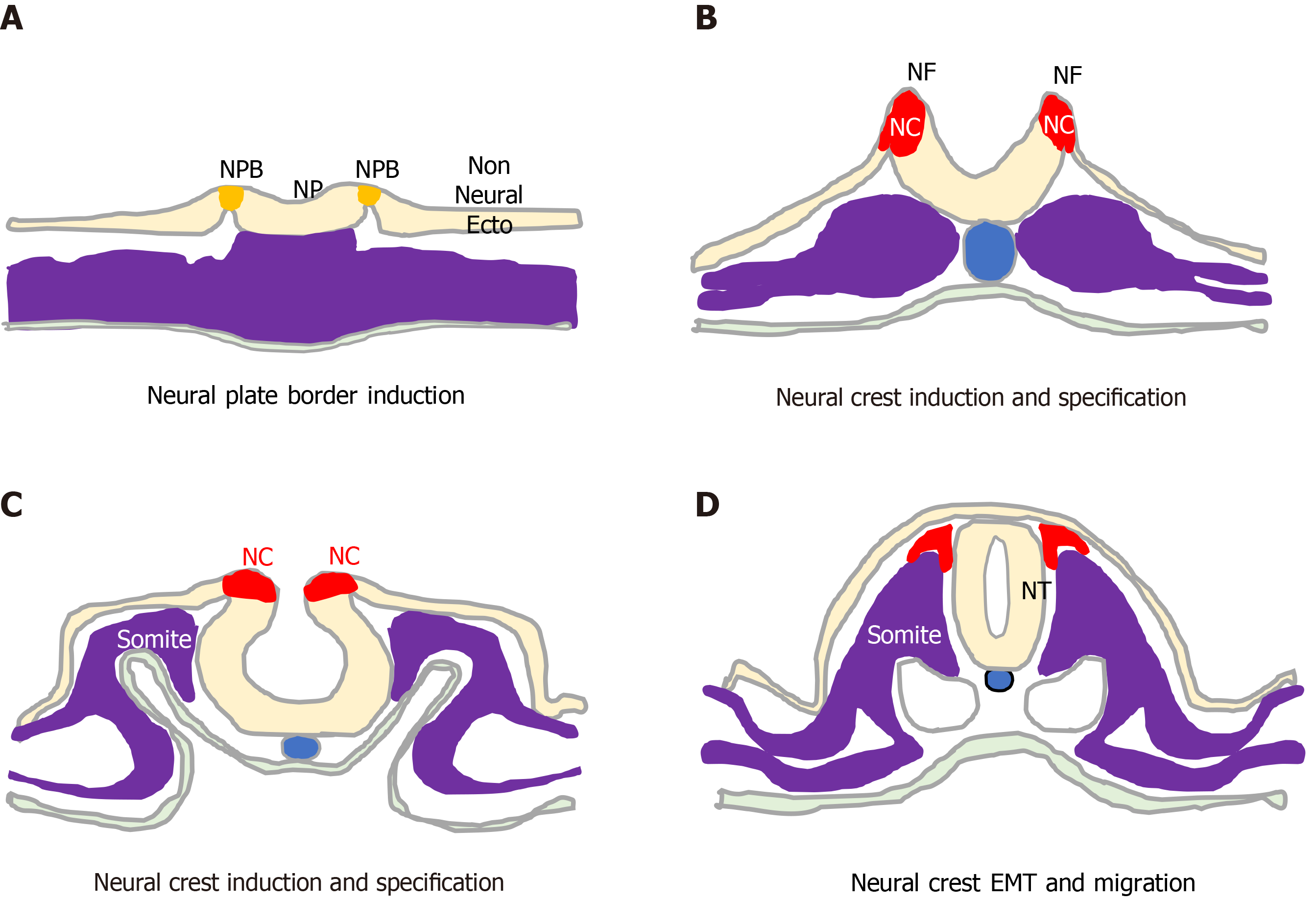
Figure 1 Neural crest formation and behaviour.
Initiation, formation and delamination of the neural crest (red). A: This process initiates during early gastrulation with specification of the neural plate border (orange) that separates the neural plate and non-neural ectoderm; B: As the neural folds form, the process proceeds through phases of neural crest (NC) induction; C: NC Specification; and D: Terminates upon neural fold closure and neural tube formation, with NC cell epithelial to mesenchymal transition, delamination and migration. NP: neural plate; NPB: neural plate border; NC: Neural crest; NF: neural fold; NT: neural tube; EMT: Epithelial to mesenchymal transition; non Neural Ecto: non-neural ectoderm.
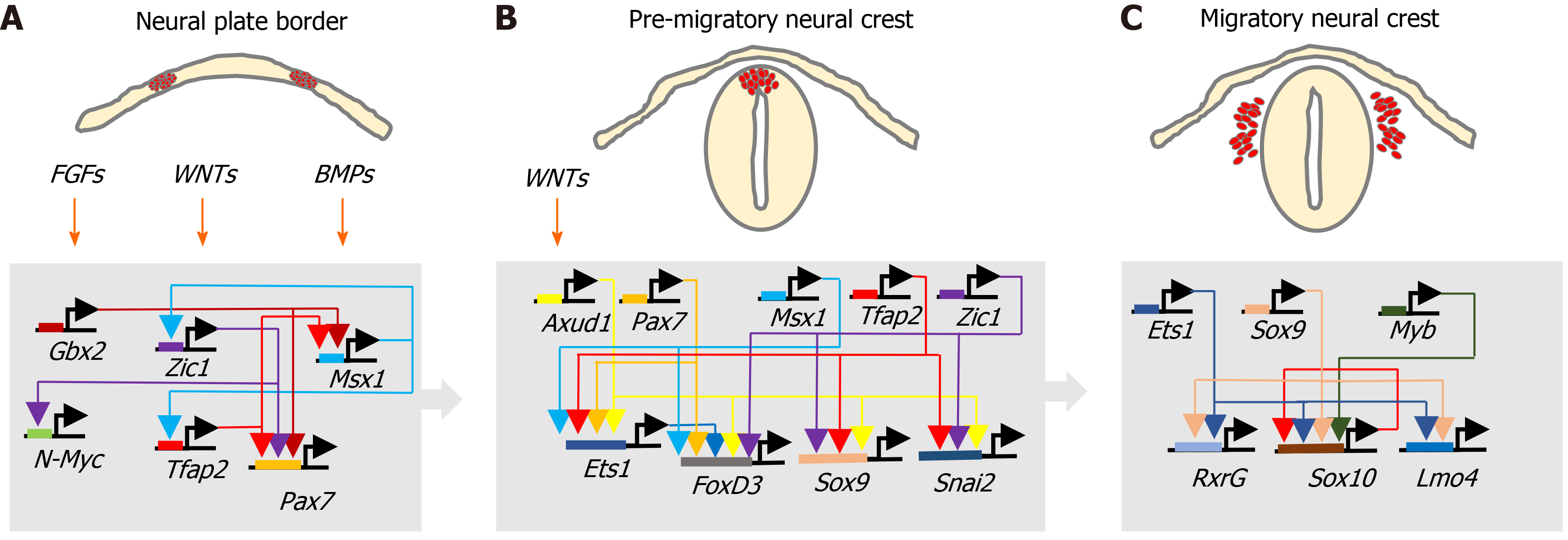
Figure 2 Neural crest transcriptional networks.
Interactions between fibroblast growth factor, wingless/Int-1 and bone morphogenic protein signalling pathways and associated transcriptional networks that regulate, A: Neural crest specification within the neural plate border; B: Induction and behaviour of the pre-migratory neural crest; and C: Migratory neural crest cell behaviour.
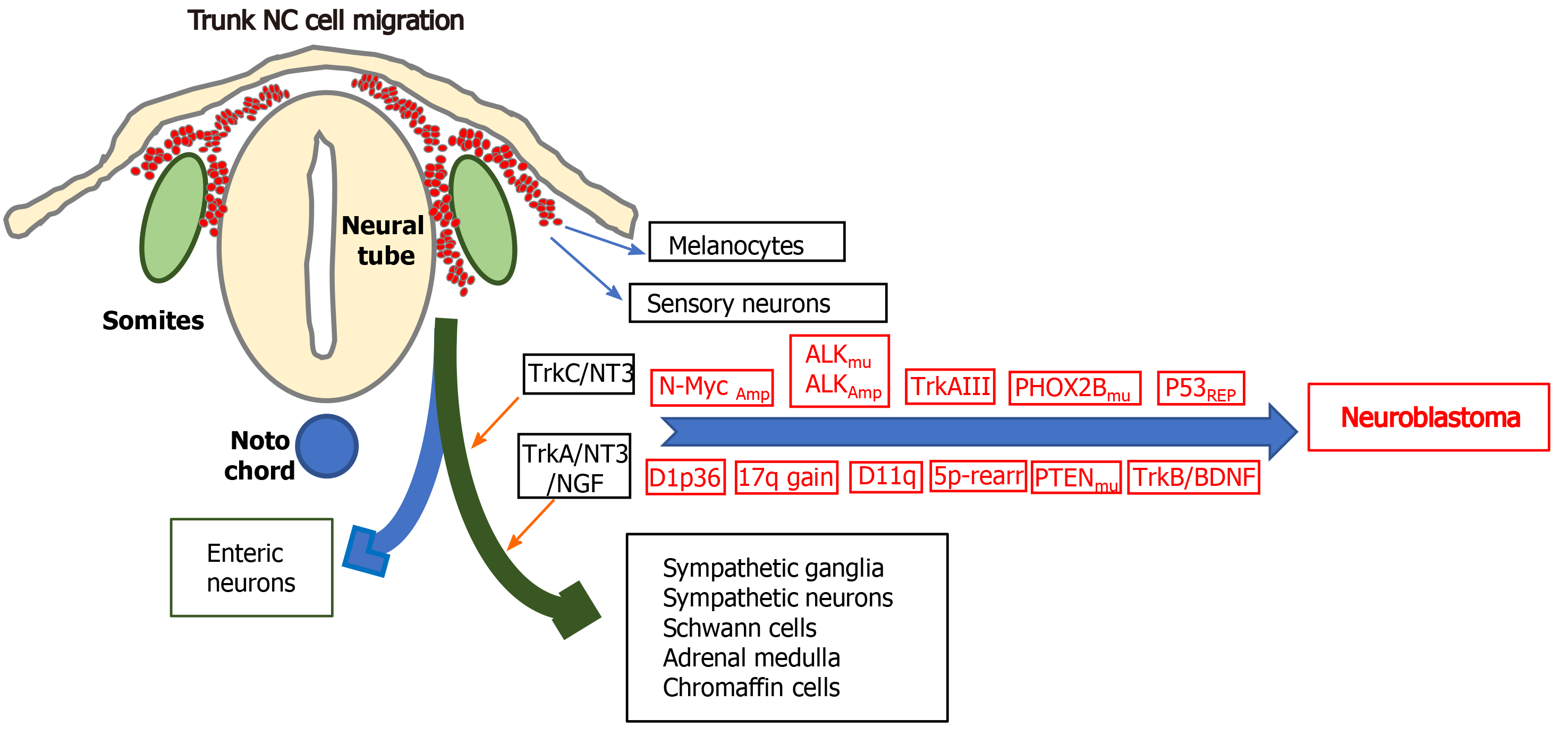
Figure 3 Genetic alterations that characterise neuroblastomas: Illustration of some of the genetic alterations, N-Myc amplification, anaplastic lymphoma kinase amplification and mutation, alternative TrkAIII splicing, p53 functional repression; chromosome 1p36 and 11q deletions; chromosome 17q gains; chromosome 5p rearrangements; mutations in PHOX2B and PTEN and brain derived neurotrophic factor/tyrosine kinase B activation, involved in neuroblastoma formation from trunk neural crest cells of the sympathoadrenal lineage, at sympathetic chain and adrenal medulla target sites, with the differentiated cell types that form from multipotent trunk neural crest cells.
NC: Neural crest; ALK: Anaplastic lymphoma kinase.
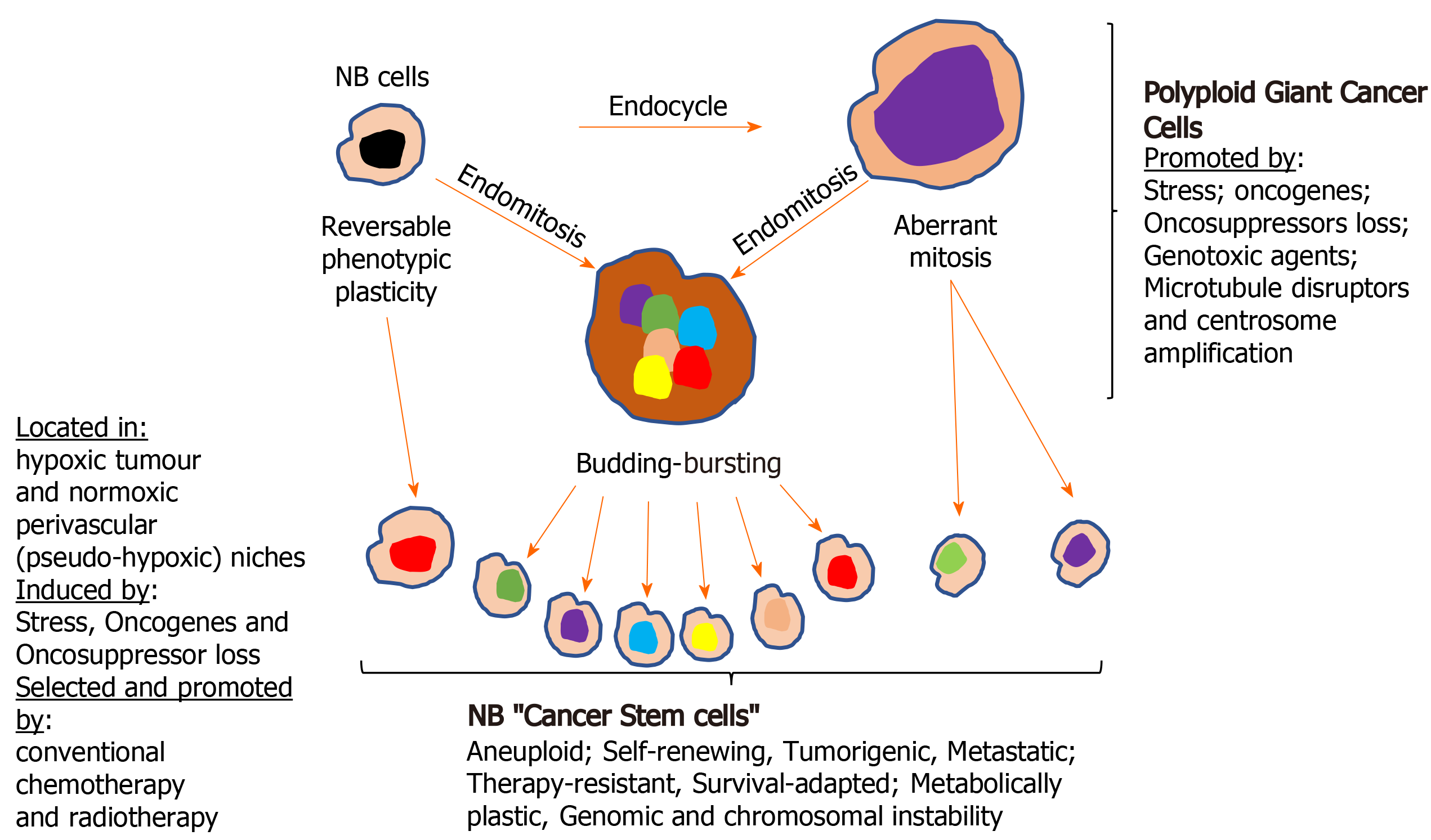
Figure 4 Formation of neuroblastoma cancer stem cells.
Illustration of the various way cancer stem cell (CSC)-like cells form in neuroblastomas, by either direct reversible phenotype plasticity from non-cancer stem cells within hypoxic tumour and pseudo-hypoxic perivascular CSC niches and/or via the formation of polyploid giant cancer cells. NB: Neuroblastomas.
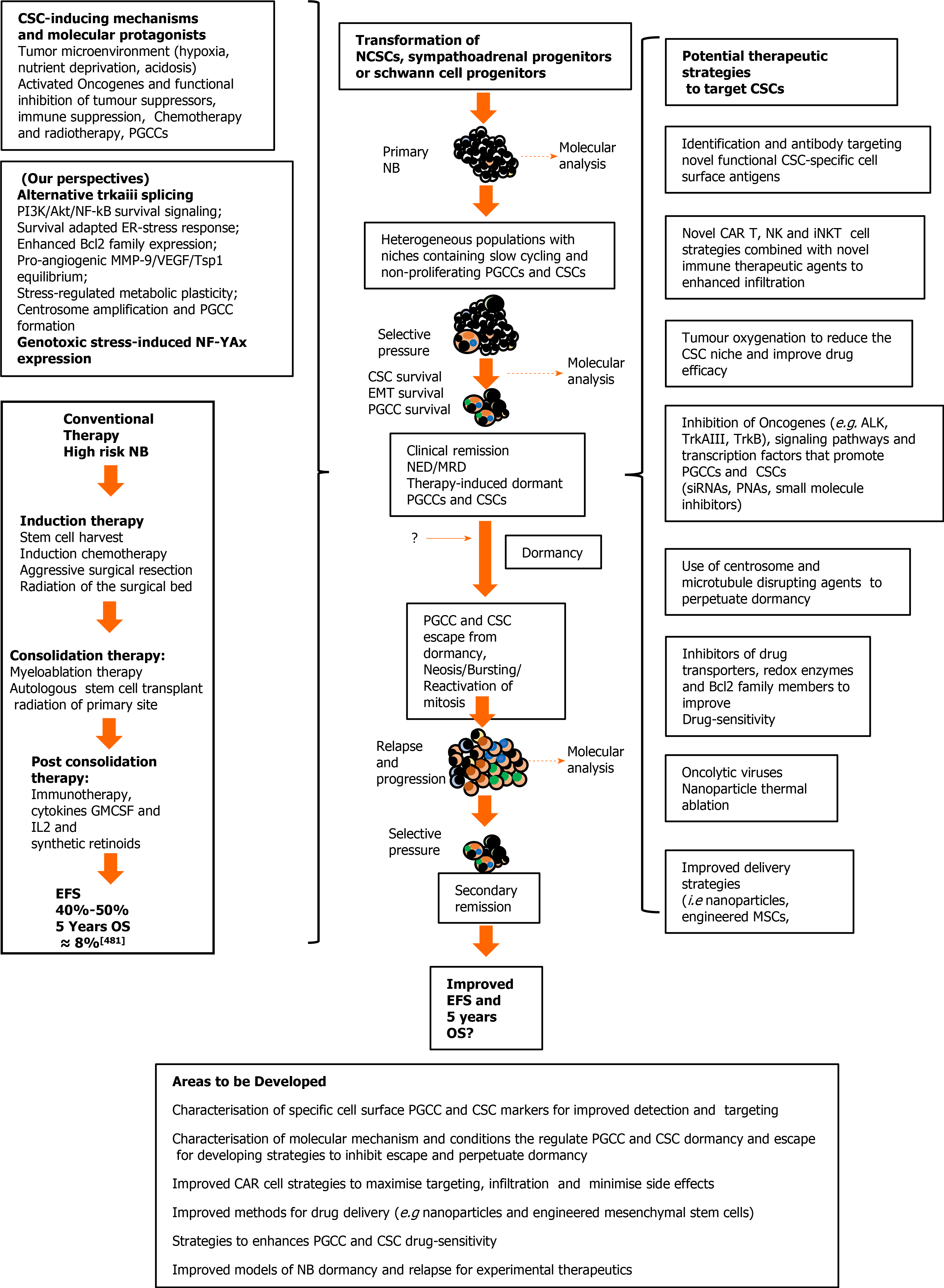
Figure 5 Cancer stem cell involvement in neuroblastoma relapse and progression, and potential therapeutic strategies.
Neuroblastomas (NB) progression (center); mechanisms, molecular protagonists and conventional therapeutic strategies that select and induce the NB polyploid giant cancer cells (PGCC) and cancer stem cells (CSC), responsible for post-therapeutic relapse and low 5-year overall survival rates, in high risk unfavourable NB (right); potential therapeutic strategies to detect, target and eliminate NB PGCC and CSC subpopulations (left), and areas requiring further development (bottom). EFS: Event free survival; OS: Overall survival; CSC: Cancer stem cell; IL-2: Interleukin 2; GMCSF: Granulocyte Macrophage Colony-Stimulating Factor; NCSC: Neural crest stem cells; NB: Neuroblastomas; NK: Natural killer cells; ALK: Anaplastic lymphoma kinase; siRNA: Small interfering ribonucleic acid; PNA: Peptide nucleic acid; MSC: Mesenchymal stem cells; PGCCs: Polyploid giant cancer cells.
- Citation: Farina AR, Cappabianca LA, Zelli V, Sebastiano M, Mackay AR. Mechanisms involved in selecting and maintaining neuroblastoma cancer stem cell populations, and perspectives for therapeutic targeting. World J Stem Cells 2021; 13(7): 685-736
- URL: https://www.wjgnet.com/1948-0210/full/v13/i7/685.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i7.685