Copyright
©The Author(s) 2021.
World J Stem Cells. May 26, 2021; 13(5): 386-415
Published online May 26, 2021. doi: 10.4252/wjsc.v13.i5.386
Published online May 26, 2021. doi: 10.4252/wjsc.v13.i5.386
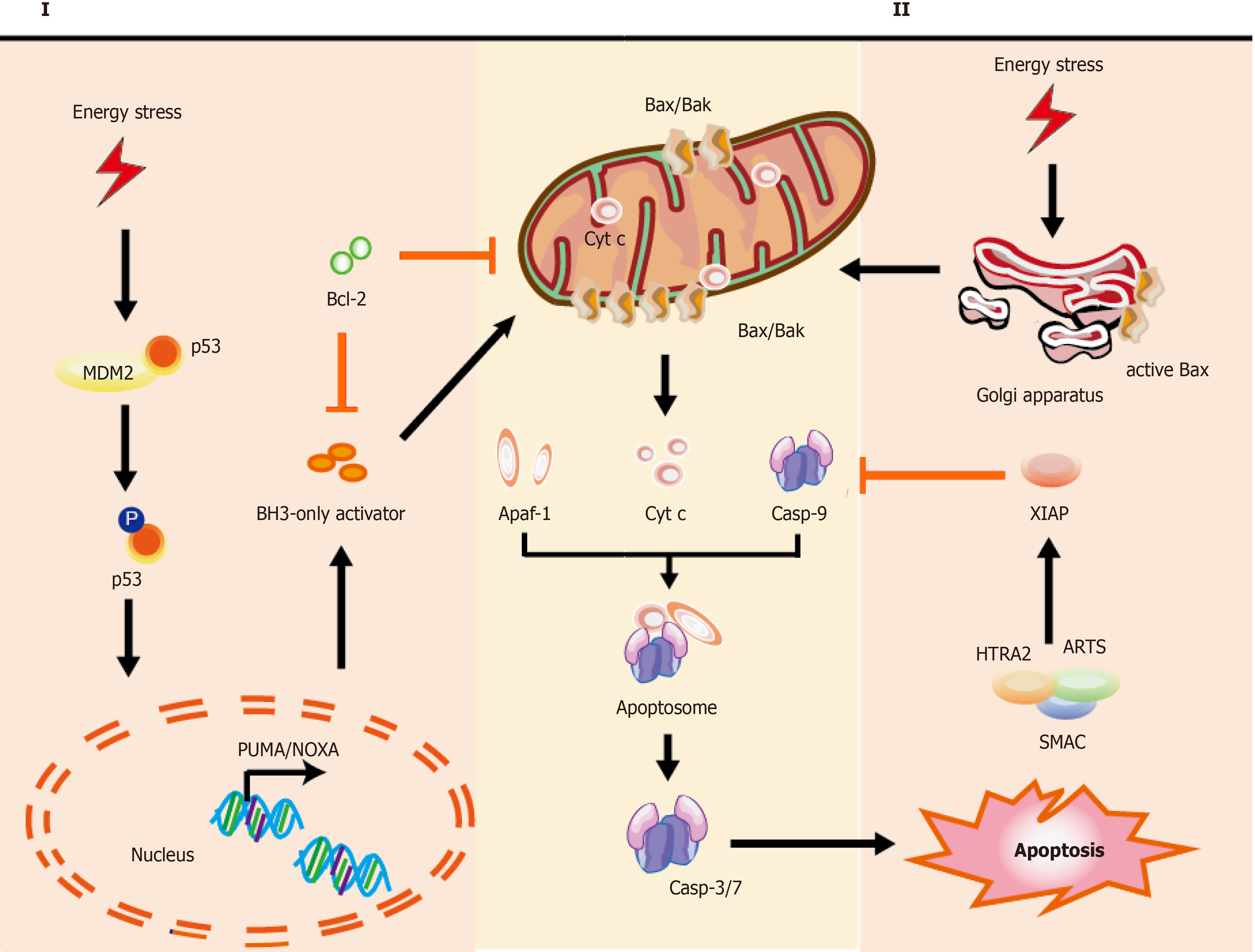
Figure 1 Mechanisms of intrinsic apoptotic pathways in stem cells.
Cell stress from various damage causes a rapid response leading to apoptosis via BH3-only activator (Way I) or active Bax directly from the Golgi (Way II) to the mitochondria, which subsequently induces a co-pathway [MOMP, cytochrome C (cyt C) releasing, etc.]. I: Stress inducers, such as DNA damage could stabilize and activate p53, which leads to p53 nuclear translocation. Subsequently, p53 exerts an impact on transcription of apoptotic genes via DNA-binding activity and its transcriptional activity (e.g., PUMA, NOXA, and Bax); II: Bax, which is monomeric in the cytoplasm, could be activated via stabilized p53 and active-Bax translocates from the Golgi to the mitochondrion. Once instigated with the apoptotic signals, active-Bax could lead to the alteration of MOMP, which undergoes dimerization and transfers to the OMM, so that relevant proteins (such as cyt C) are released into the cytosol usually confined in the intermembrane space. The released cyt C is involved in apoptosome formation via binding to the cytosolic Apaf-1. This complex recruits and activates initiator pro-casp-9, and then act-casp-9 activates downstream executor casp-3/-6/-7, leading to apoptotic cell death. In the cytoplasm, IAP antagonists (e.g., SMAC, ARTS, and HTRA2) could bind and suppress XIAP, causing the activation of casp-9 for the apoptotic pathway. The T-shaped lines indicate inhibitory interactions involved in this pathway, while the solid arrows indicate activating interactions. Bax: Apoptosis regulator Bcl-2 associated X protein; OMM: Outer membrane permeabilization; MOMP: Mitochondrial outer membrane permeabilization, cyt C: Cytochrome C; PUMA: p53 upregulated modulator of apoptosis, NOXA: Pro-apoptotic BH3-only protein, also known as PMAIP1, phorbol-12-myristate-13-acetate-induced protein 1; Apaf-1: Apoptosis protease activating factor-1; IAP: Inhibitor of apoptosis; SMAC: Second mitochondria-derived activator of caspase; ARTS: Apoptosis-related protein in the transforming growth factor-β signaling pathway; HTRA2: High-temperature-required protein A2.
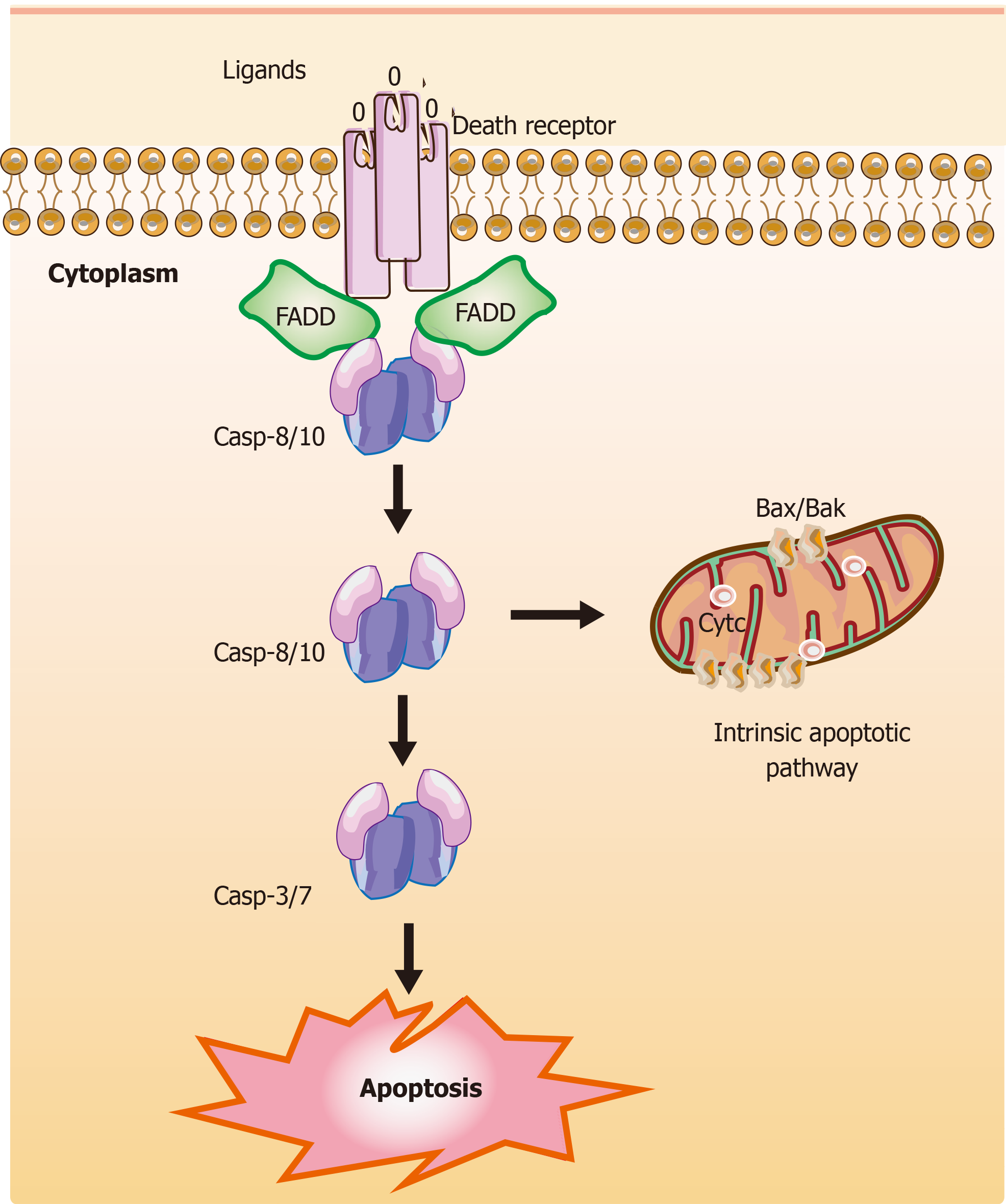
Figure 2 Mechanisms of extrinsic apoptotic pathways in stem cells.
The extrinsic apoptotic pathway (also known as the death receptor-dependent pathway) is induced by the connection between death receptors exposed on the cell surface [tumor necrosis factor (TNF) receptor] and the specific TNF family ligands. Subsequently, this signaling causes a conformational change leading to the recruitment of Fas-associated death domain (FADD) and allows interactions between FADD and casp-8 and/or the casp-10, resulting in the cleavage and activation of casp-3 and casp-7 through their death domain. Finally, the active and cleaved casp-3 induces changes in phosphatidylserine exposure, DNA fragmentation, and the formation of apoptotic bodies. Also, casp-8 can target the BH3-only protein Bid and cleave Bid to a truncated fragment t-Bid, which could connect to the extrinsic apoptotic pathways. The T-shaped lines indicate inhibitory interactions involved in this pathway, while the solid arrows indicate activating interactions. FADD: Fas-associated death domain.
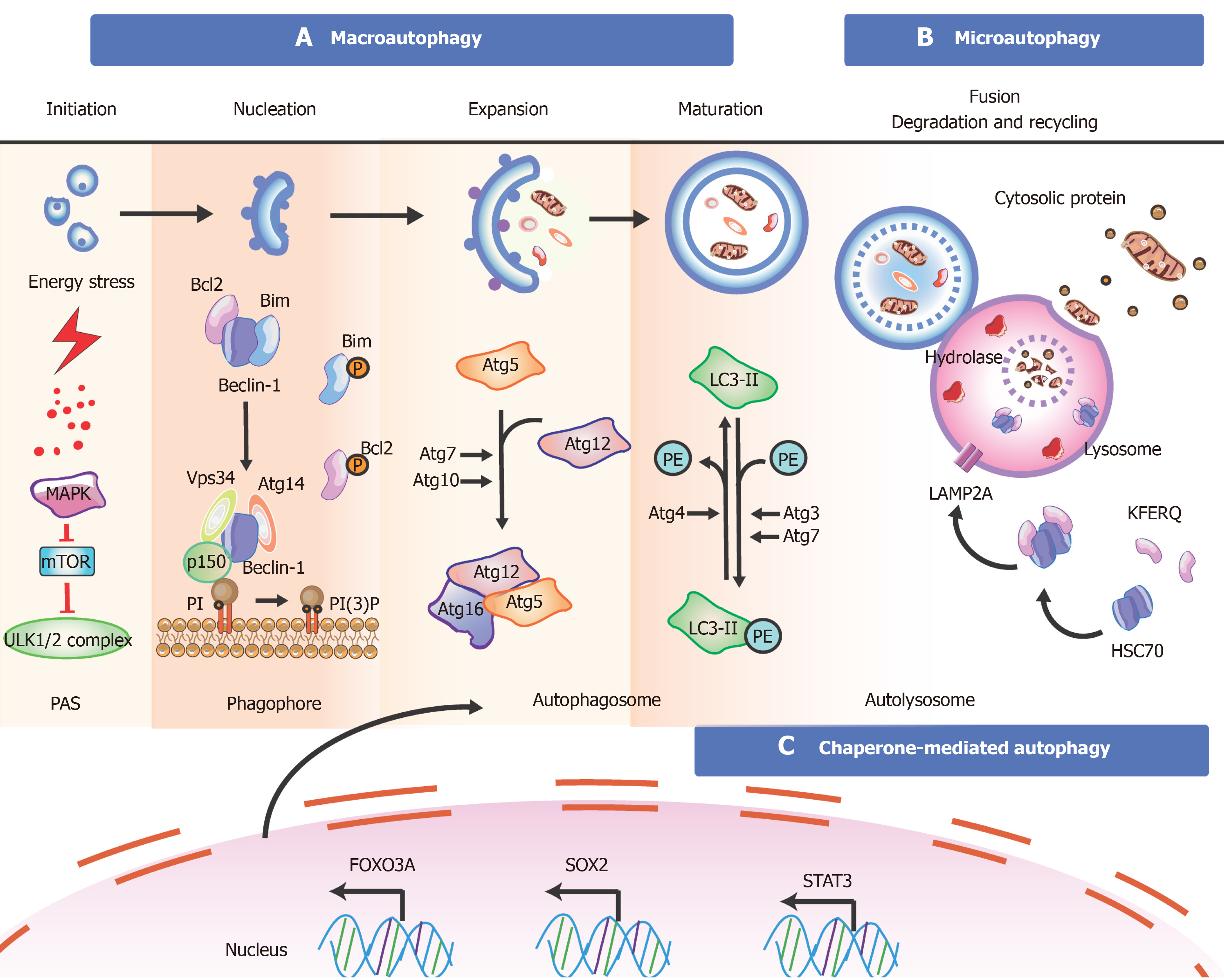
Figure 3 Overview of the mechanisms during autophagy in stem cells.
There are three types of autophagy [macroautophagy (section a), microautophagy (section b), and chaperone-mediated autophagy (section c)] based on different pathways; however, they produce the same results. Besides these proteins, key transcription factors closely related to autophagy are shown. The T-shaped lines indicate inhibitory interactions involved in this pathway, while the solid arrows indicate activating interactions. A: Typically, the mTORC1 complex functions as an inhibitor to control the initiation of autophagy. Under environmental stresses and physiological stressors, AMPK is activated to inhibit the activity of mTORC1, leading to a release of the ULK1 (Unc-51-like kinase complex, also known as ATG1) complex to induce autophagy. This initiation process is known as the phagophore assembly site (PAS) formation. Next, PI3 is phosphorylated to PI3P via the class III PI3-kinase-Beclin1 complex formed by core subunits of Beclin1 (Atg6), Atg14 L, and Vps34-Vps15, resulting in autophagosome formation. The Atg12-Atg5-Atg16L1 complex acts as a regulator for enveloping and translocating the cytoplasmic cargo to the lysosome in misfolded-protein degradation. Atg4 can cleave LC3 (Atg8) to generate cytosolic LC3-I. Atg3 (E2 enzymes) and Atg7 (E1-like enzymes) can lead the conjugation of PE to LC3-I to form lipidated LC3-II, which is combined with the autophagosome membrane to complete and elongate autophagosome formation. Finally, the autophagosome contents undergo degradation due to low lysosomal pH; B: In microautophagy, misfolded or/and toxic proteins can be directly engulfed by the lysosomal membrane and degraded in the lysosome; C: During chaperone-mediated autophagy, the heat shock cognate 70 kDa protein (HSC70) chaperones attach to the pentapeptide motif KFERQ (namely Lys-Phe-Glu-Arg-Gln) for delivery to lysosomes via a specific receptor LAMP2A. Also, some of the key transcription factors are closely linked to the stem cell state and the occurrence of autophagy (bottom). FOXO3A can enhance autophagosome formation via autophagy gene expression in hematopoietic stem cells and breast cancer stem-like cells, which is needed to mitigate an energy crisis and allow cell survival. Besides FOXO3A, other transcription factors such as SOX2, STAT3, OCT4, KLF4, and c-Myc are also vital for reprogramming in the initial creation of stem cells at the genetic level during autophagy.
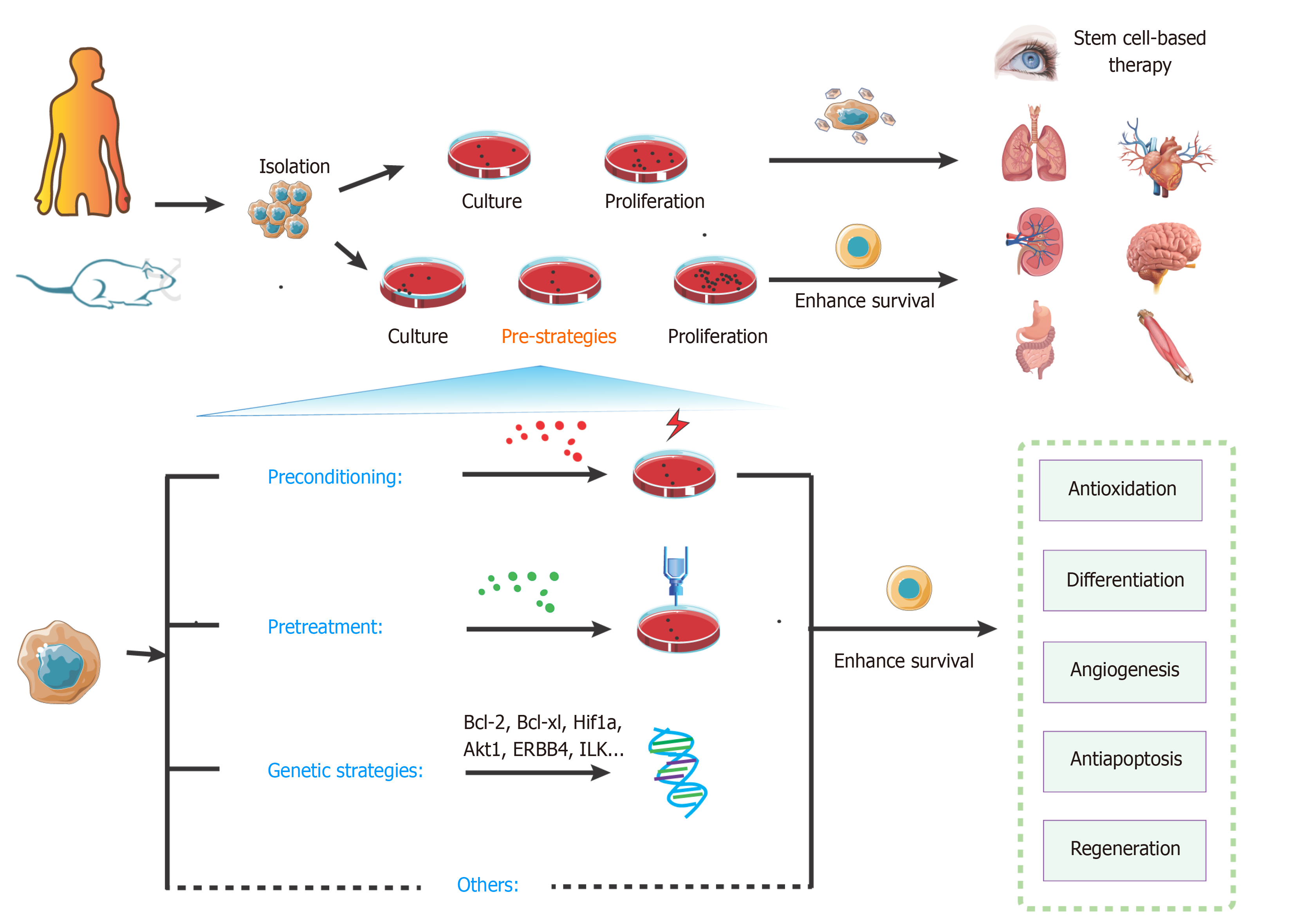
Figure 4 Overview of key strategies to enhance stem cell transplantation therapy.
The steps of stem cell-based transplantation therapy include drawing the materials, isolation, culture, proliferation, and transplantation. Compared with the classic approaches, pre-strategies could enhance survival of stem cells. These pre-strategies mainly include preconditioning, pretreatment, genetic strategies, and other methods. They can effectively activate various signaling pathways for protecting cells from injury and promoting survival.
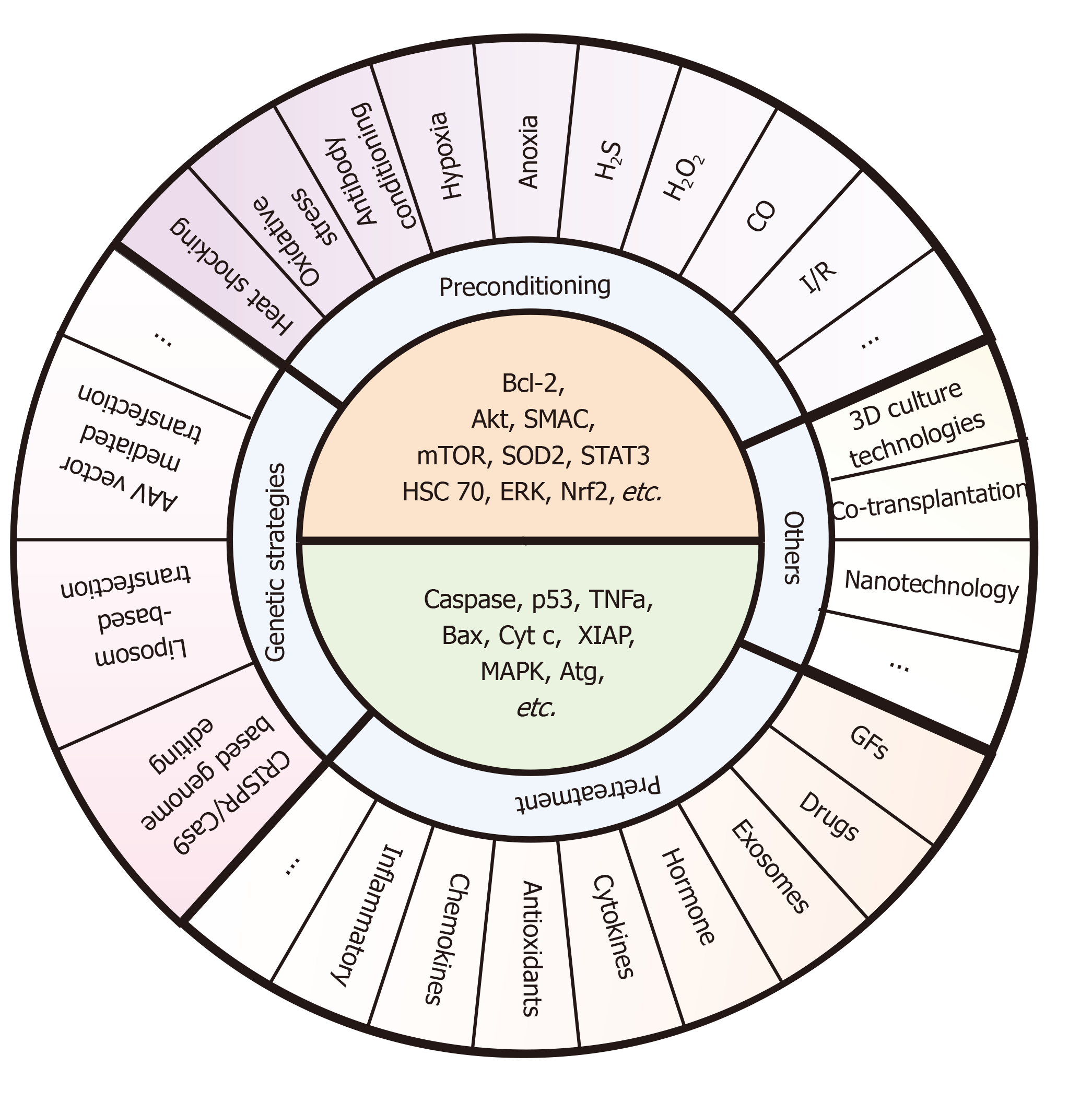
Figure 5 Specific pre-strategies and their key molecule targets for enhancing stem cell transplantation therapy.
These pre-strategies mainly include preconditioning (e.g., exposure to oxidative stress, heat shock, and ischemic/hypoxic injury), pretreatment (e.g., drug treatment, cytokines, antioxidants, nitric oxide, glucose deprivation, growth factors, miRNAs, and exosomes), genetic strategies (e.g., AAV vector mediated transfection, Liposome-based transfection, and CRISPR/Cas9-based genome editing), and other methods (e.g., 3D culture technologies, co-transplantation, and nanotechnology). The core ideas of these pre-strategies are to upregulate the survival factors (e.g., Bcl-2, Akt, SMAC, mTOR, SOD2, STAT3, HSC 70, ERK, and Nrf2) and downregulate the death catalyzers (e.g., caspase, p53, TNFa, Bax, Cyt c, XIAP, MAPK, and Atg). However, there are few methods targeting all of these molecules at the same time during the co-network. Also, studies pay more attention to certain signaling such as Bcl-2 and mTOR, and other signals such as Atg or XIAP still need further mining.
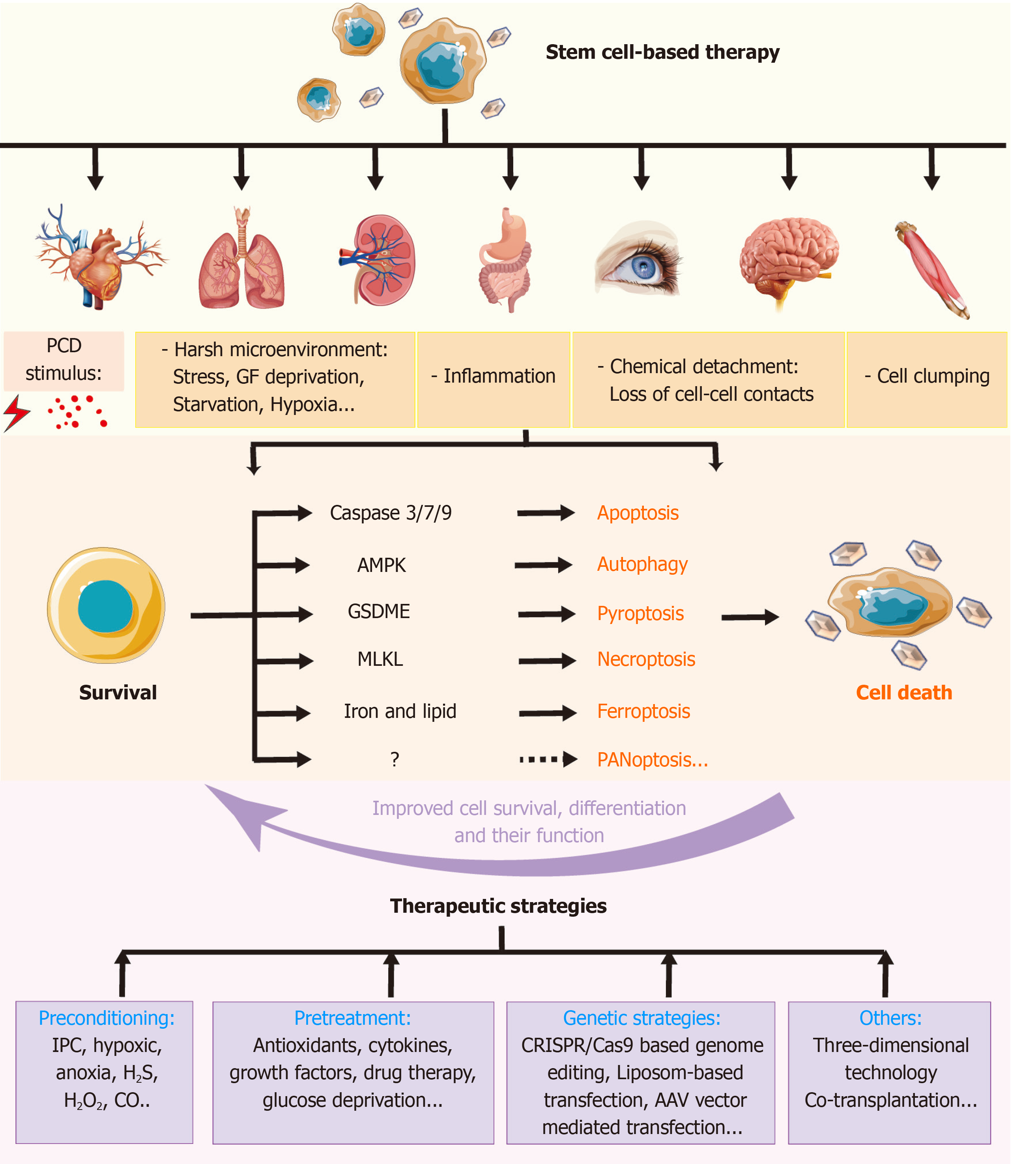
Figure 6 Role of regulated cell deaths in stem cell-based transplantation and therapeutic pre-strategies to improve the therapy.
Stem cell-based therapy has been used in various diseases. A number of stimuli may induce regulated cell deaths (RCDs) in transplanted stem cells (SCs), which results in poorer outcomes. Different signals involved in distinct types of RCDs may provide some targets to improve SC-based transplantation. These therapeutic strategies include preconditioning, pretreatment, gene strategies, and so on. IPC: Ischemic preconditioning; PCD: Programmed cell death; MLKL: Mixed lineage kinase domain like protein; GSDME: Gasdermin E.
- Citation: Hu XM, Zhang Q, Zhou RX, Wu YL, Li ZX, Zhang DY, Yang YC, Yang RH, Hu YJ, Xiong K. Programmed cell death in stem cell-based therapy: Mechanisms and clinical applications. World J Stem Cells 2021; 13(5): 386-415
- URL: https://www.wjgnet.com/1948-0210/full/v13/i5/386.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i5.386