Copyright
©The Author(s) 2020.
World J Stem Cells. May 26, 2020; 12(5): 303-322
Published online May 26, 2020. doi: 10.4252/wjsc.v12.i5.303
Published online May 26, 2020. doi: 10.4252/wjsc.v12.i5.303
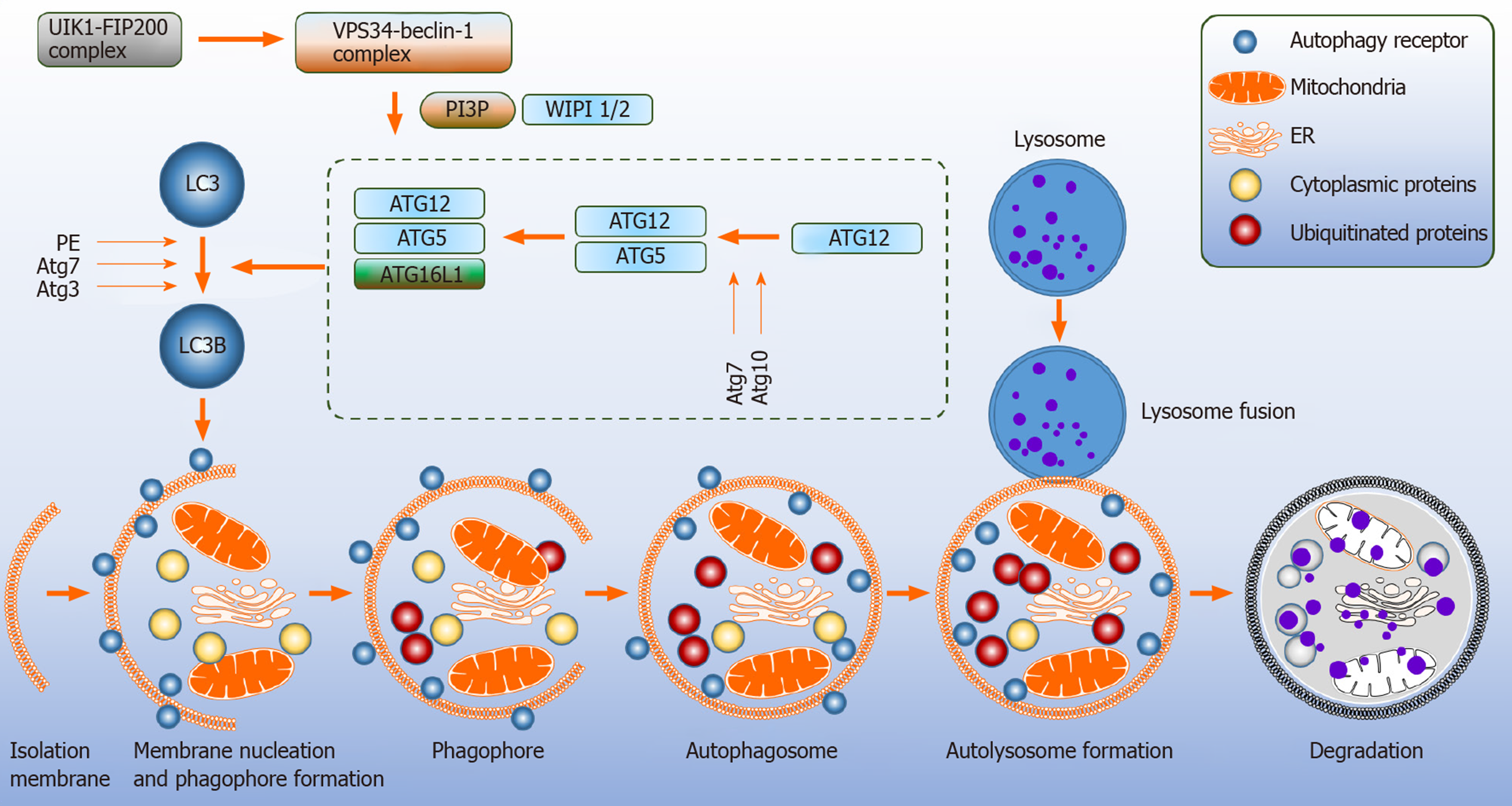
Figure 1 Canonical autophagy pathway.
Autophagy is a multistep process that includes the following steps: initiation, nucleation, elongation, maturation and fusion with the lysosome. Several proteins referred to as autophagy related genes regulate this process. Autophagy is stimulated under basal conditions and is induced by stress, for example nutrient deprivation. ATG: Autophagy related genes; ER: Endothelial reticulum; FIP200: FAK family kinase interacting protein of 200 kDa; LC3: Light chain 3; PE: Phosphatidylethanolamine; PI3P: Phosphatidylinositol-3-phospate 3; ULK1: Unc-51-like kinase 1; VPS34: Vacuole protein sorting 34.
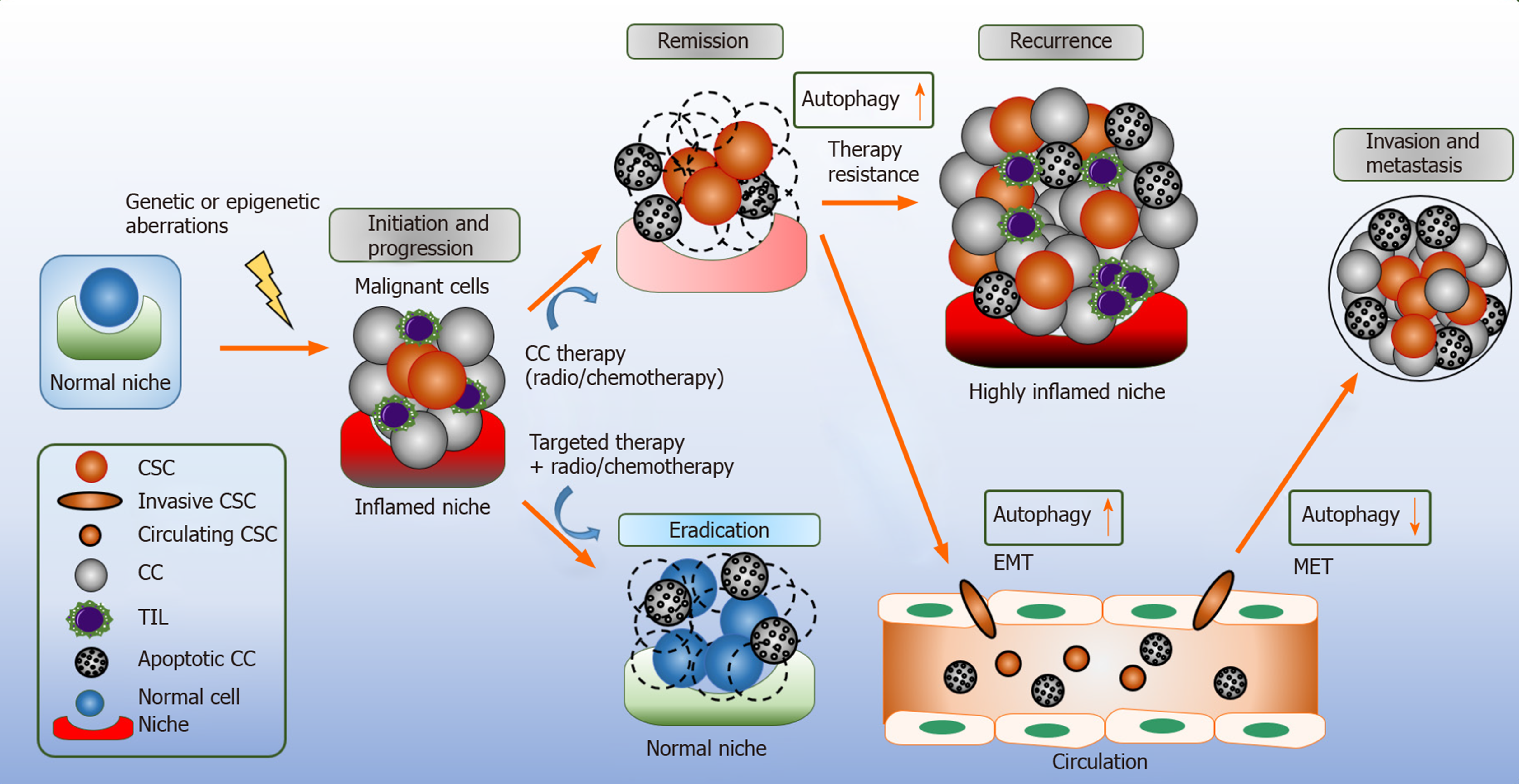
Figure 2 Autophagy in cancer stem cells.
Autophagy has a context dependent role in cancer. Cancer stem cells (CSCs) are a heterogeneous collection of different cells types that acquire genetic aberrations/epigenetic modifications and retain the ability to undergo extensive cell proliferation, retain stemness and give rise to differentiated diverse cancer cell lineages. Potentially the CSC niche will provide protective mechanisms for the disease propagation. Autophagy promotes invasion of cancer stem cells through TGF-1β dependent epithelial-mesenchymal transition; however, during mesenchymal-epithelial transition autophagy is downregulated as the circulating CSCs are scavenging an organ to seed for metastasis. Moreover, autophagy reinforces the resilience of CSCs plasticity, remodeling the immunosurveillance and facilitating the acquisition of resistance to conventional chemotherapies which contribute to cancer relapse. By targeting autophagy, cancer cells and CSCs are sensitized to enhancing the efficacy of chemotherapy agents and reducing their toxicity and disease relapse. CSC: Cancer stem cell; CC: Cancer cell; EMT: Epithelial-mesenchymal transition; MET: Mesenchymal-epithelial transition; TIL: Tumor-infiltrating lymphocytes.
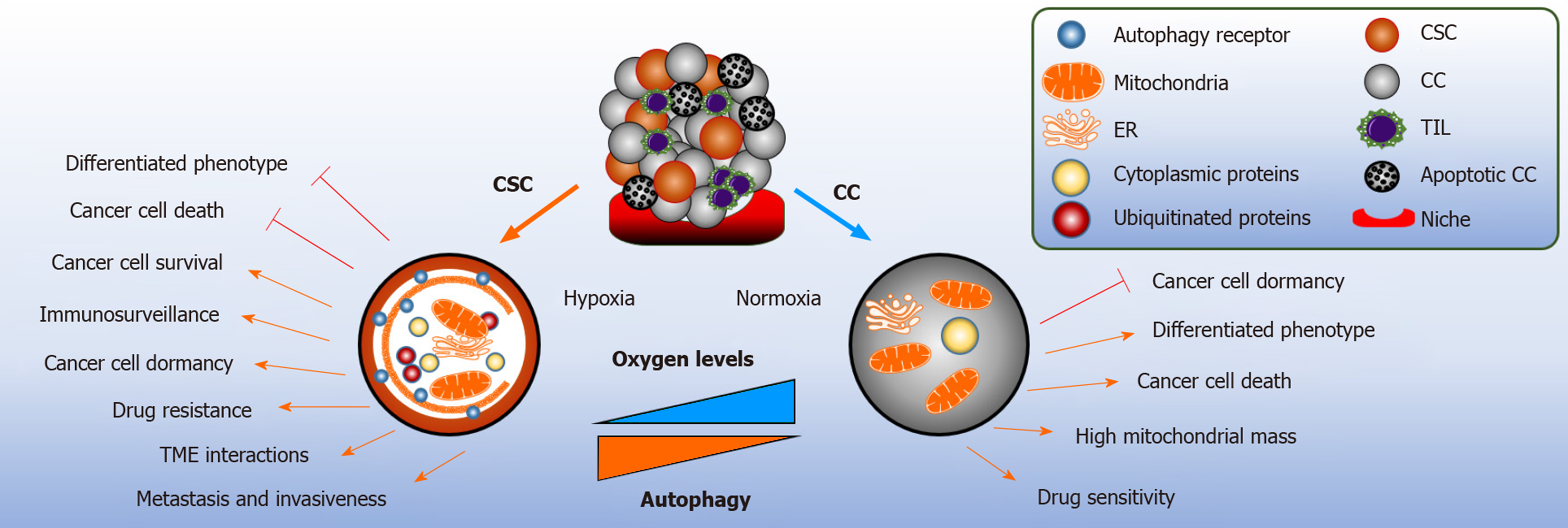
Figure 3 The divergent role of autophagy in cancer stem cells and cancer cells.
Cancer stem cells (CSCs) drive the initiation and progression of cancer in multiple tumors. CSCs are reliant on their niches to sustain their self-renewal capacity and plasticity. Hypoxia induced autophagy, provides metabolic plasticity to CSCs. The role of autophagy in hypoxia is to modulate the metabolic remodeling of cancer cells, in particular CSCs. Additionally, autophagy and hypoxia have been implicated in immunosurveillance of CSCs during glucose limitation by increasing the expression of programmed death ligand 1 which results to tumor-infiltrating lymphocytes exhaustion. In addition, autophagy supports tumor dormancy, metastasis and invasion resulting to the treatment of resistant CSCs. CSC: Cancer stem cell; CC: Cancer cell; ER: Endothelial reticulum; TIL: Tumor-infiltrating lymphocytes; TME: Tumor microenvironment.
- Citation: Mandhair HK, Arambasic M, Novak U, Radpour R. Molecular modulation of autophagy: New venture to target resistant cancer stem cells. World J Stem Cells 2020; 12(5): 303-322
- URL: https://www.wjgnet.com/1948-0210/full/v12/i5/303.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i5.303