Copyright
©The Author(s) 2020.
World J Stem Cells. Jan 26, 2020; 12(1): 8-24
Published online Jan 26, 2020. doi: 10.4252/wjsc.v12.i1.8
Published online Jan 26, 2020. doi: 10.4252/wjsc.v12.i1.8
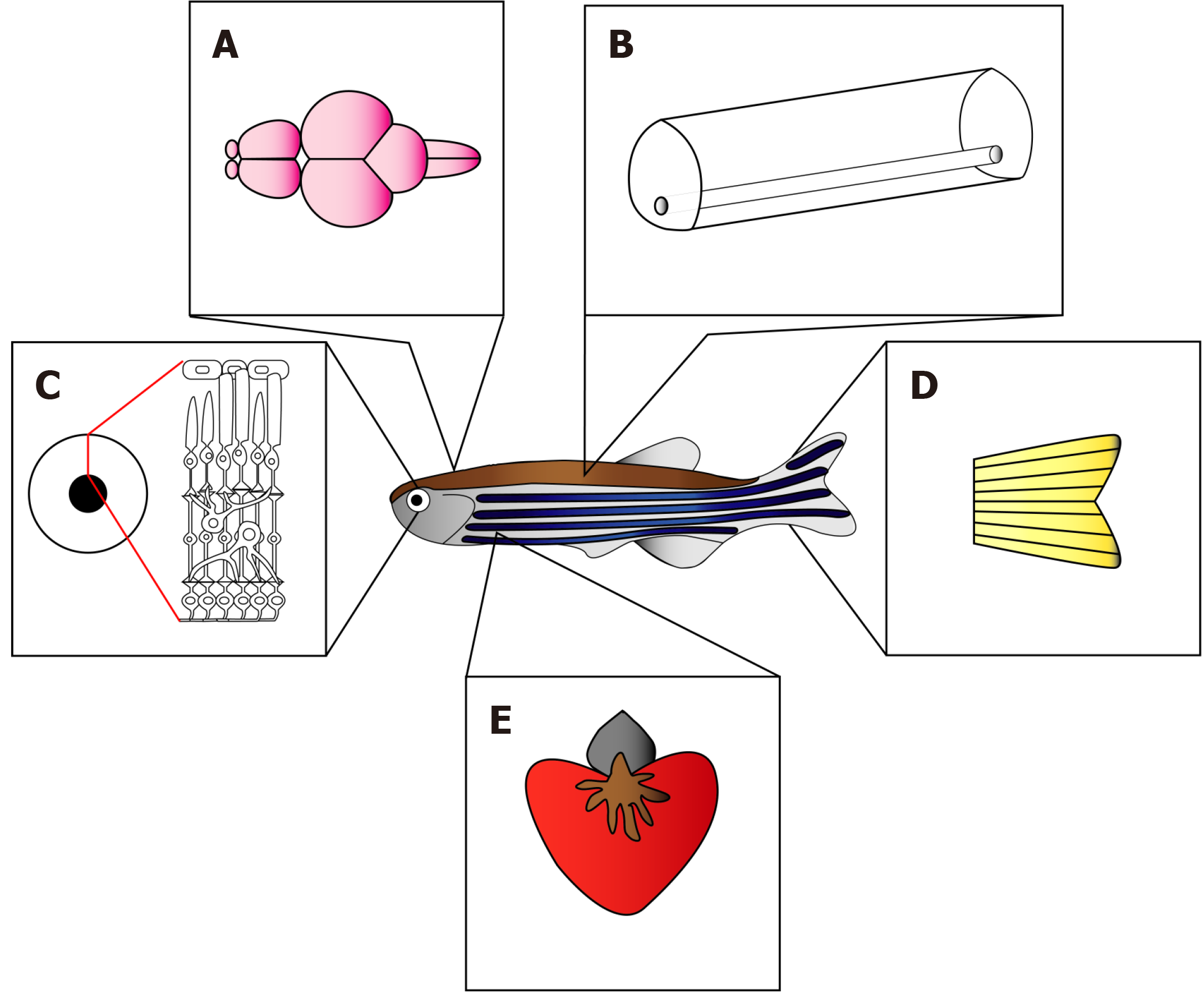
Figure 1 Regenerating organs in adult zebrafish.
In contrast to mammals, adult zebrafish are able to efficiently regenerate the lost tissue architecture and retrieve the functions of brain (A), spinal cord (B), retina (C), fin (D) and heart (E).
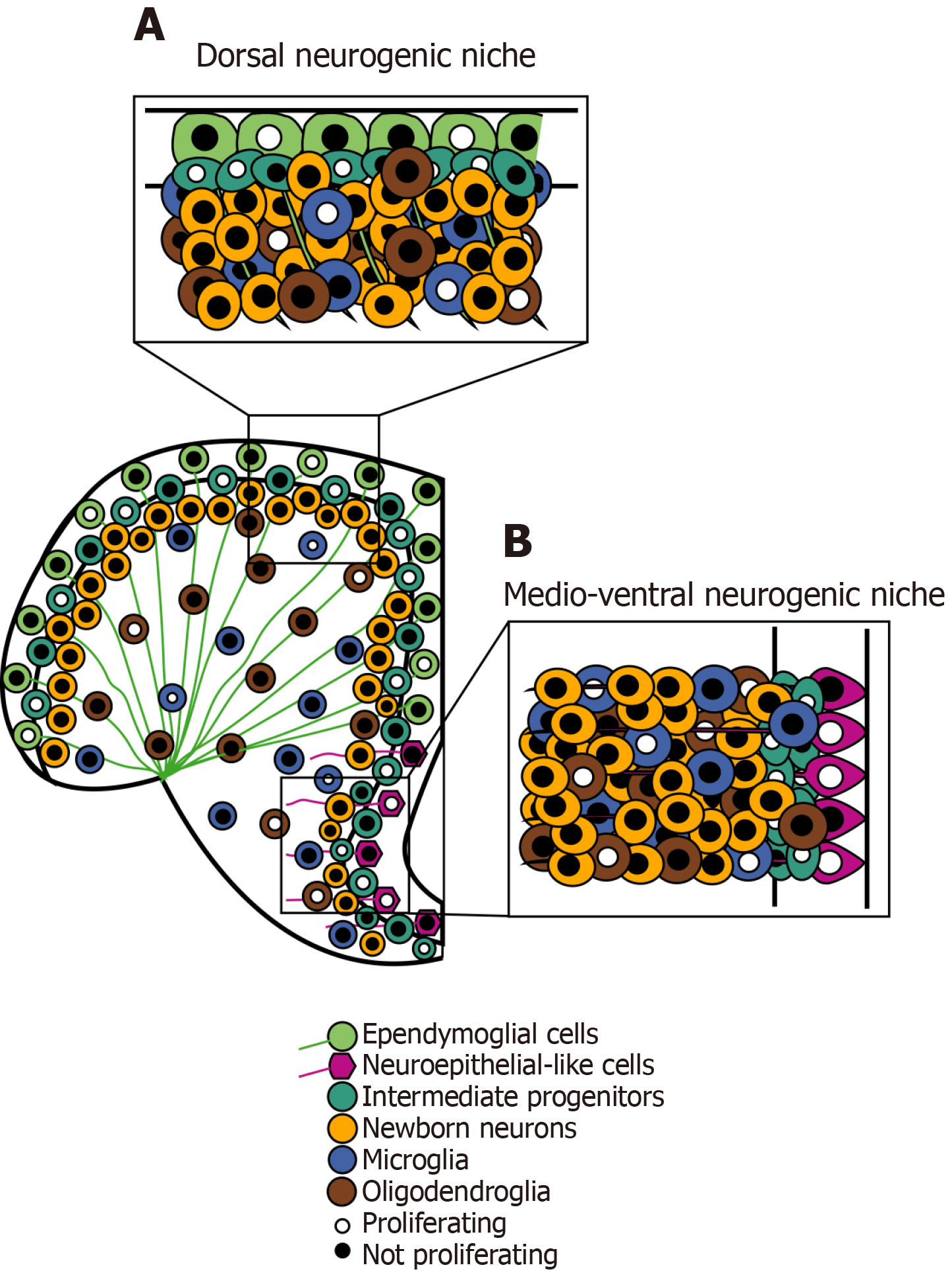
Figure 2 Schematic representation of the main cell types in the adult zebrafish telencephalon, with focus on two distinct neurogenic niches, the dorsal ventricular zone and the medio-ventricular zone.
A: The dorsal ventricular zone hosts ependymoglial cells (light green), quiescent and slow-cycling adult neural stem cells, intermediate progenitor cells (light blue) and neurons (yellow); B: The medio-ventricular zone hosts neuroepithelial-like cells (magenta), characterized by faster cell cycle. Intermediate progenitor cells (light blue) are deposited in the subventricular layer and they can either differentiate into neurons (yellow) or migrate to the olfactoy bulb. Microglial (blue) and oligodendroglial (brown) cells can be found in the subventricular zone and in the parenchyma of the adult zebrafish telencephalon.
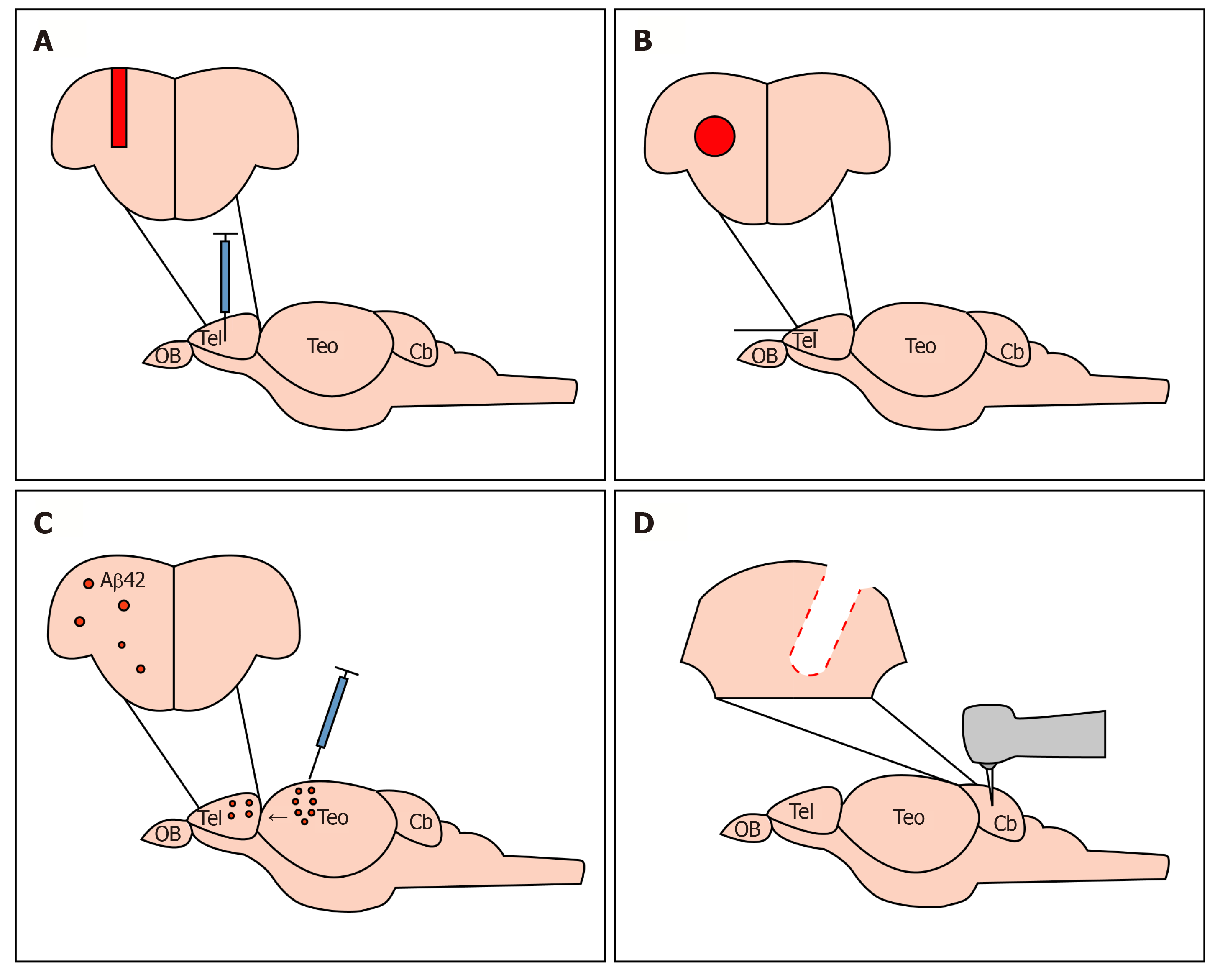
Figure 3 Established paradigms to study cellular and molecular mechanisms of regeneration in the telencephalon and cerebellum of adult zebrafish.
A: Mechanical injury to lesion the adult zebrafish telencephalon, damaging the ventricular zone containing neural stem cells; B: Mechanical injury to lesion the adult zebrafish telencephalon, sparing the ventricular zone containing neural stem cells; C: Cerebroventricular microinjections of Aβ42 derivatives to study neurodegeneration in the adult zebrafish telencephalon; D: Mechanical injury of the adult zebrafish cerebellum.
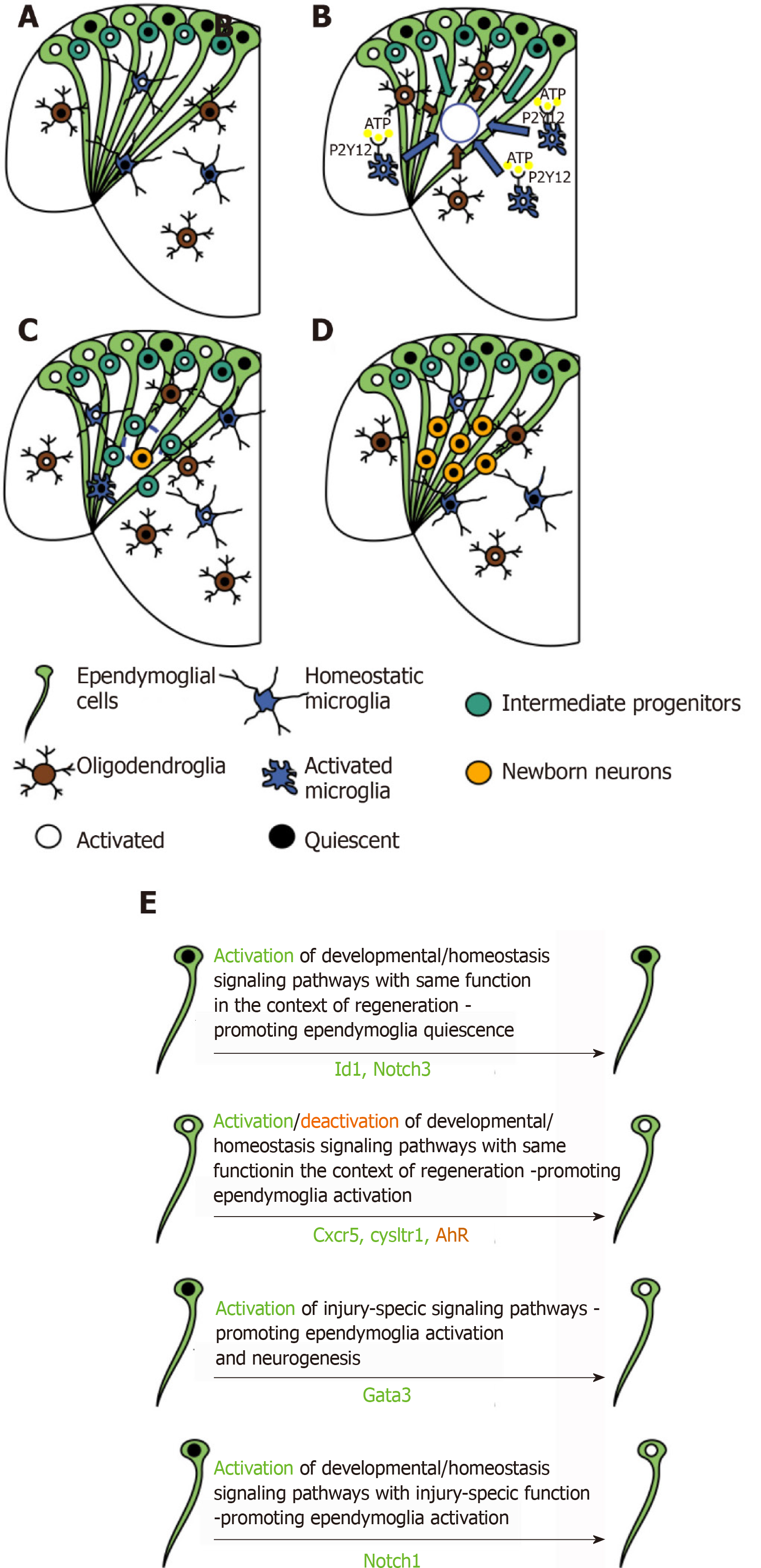
Figure 4 Glial cell reactivity and tissue restoration in the adult zebrafish telencephalon in response to stab wound injury.
A: Representative scheme of cell composition in the intact telencephalon of adult zebrafish; B-D: Cellular response to mechanical injury in the adult zebrafish telencephalon; B: Injury-induced cell death stimulates the activation of inflammatory response. ATP is sensed by microglial cells (blue), through P2Y12 receptor, triggering their change in morphology and their migration, together with oligodendroglial precursor cells (OPCs, brown) at the injury site (blue circle); C: Microglial and oligodendroglial cell accumulation is resolved and intermediate progenitors (light blue), source of newly formed neurons (yellow), populate the injury site, where they start differentiating into neurons. Proliferation reaches its peak in the ventricular zone, where stem cells (ependymoglial cells, light green) reside. This cellular response seems to be required to increase the neuronal output and to re-populate the loss of progenitors in the subventricular zone, due to their migration to compensate the neuronal loss at the injury site; D: Regeneration is efficiently completed, stem cell activation returns to basal levels, surviving newborn neurons are found at the injury site and manage to survive, no signs of glial scar can be found; E: Scheme of genes specifically regulated in ependymoglial cells, relevant for their activation state and the promotion of restorative neurogenesis.
- Citation: Zambusi A, Ninkovic J. Regeneration of the central nervous system-principles from brain regeneration in adult zebrafish. World J Stem Cells 2020; 12(1): 8-24
- URL: https://www.wjgnet.com/1948-0210/full/v12/i1/8.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i1.8