Copyright
©The Author(s) 2019.
World J Stem Cells. Aug 26, 2019; 11(8): 452-463
Published online Aug 26, 2019. doi: 10.4252/wjsc.v11.i8.452
Published online Aug 26, 2019. doi: 10.4252/wjsc.v11.i8.452
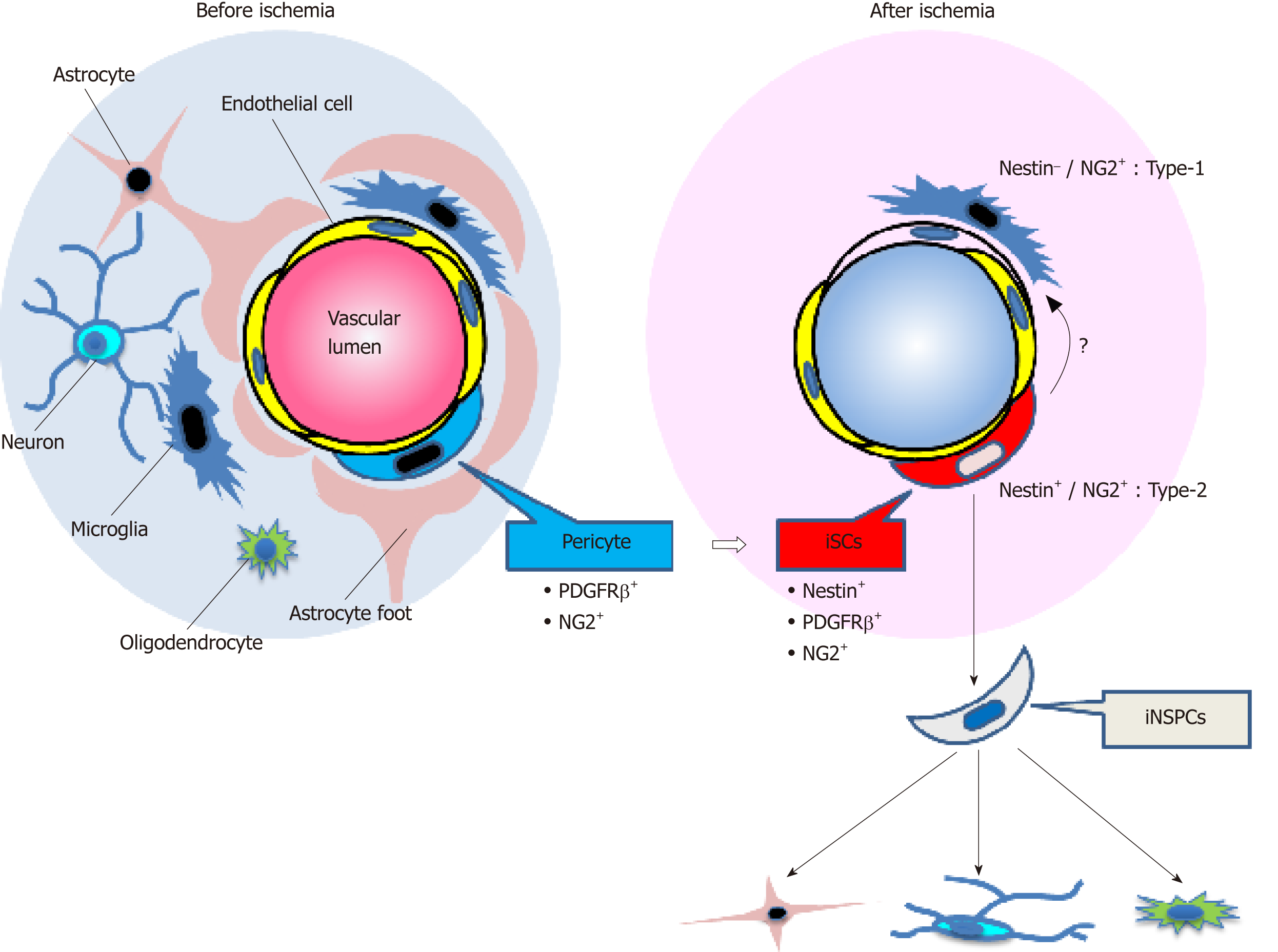
Figure 1 Schematic representation of the fate of injury/ischemia-induced multipotent stem cells and injury/ischemia-induced neural stem/progenitor cells following ischemic stroke.
Under ischemic conditions following stroke, brain pericytes, which constitute the neurovascular unit together with endothelial cells and neural lineage cells, may convert into induced multipotent stem cells (iSCs) by acquiring stemness. iSCs may generate induced neural stem/progenitor cells, which have the potential to differentiate into various neural lineage cells, including neurons, astrocytes, and oligodendrocytes. NG2: Neuronal/glial 2; iSCs: Injury/ischemia-induced multipotent stem cells; PDGFRβ: Platelet-derived growth factor receptor beta; iNSPCs: Injury/ischemia-induced neural stem/progenitor cells.
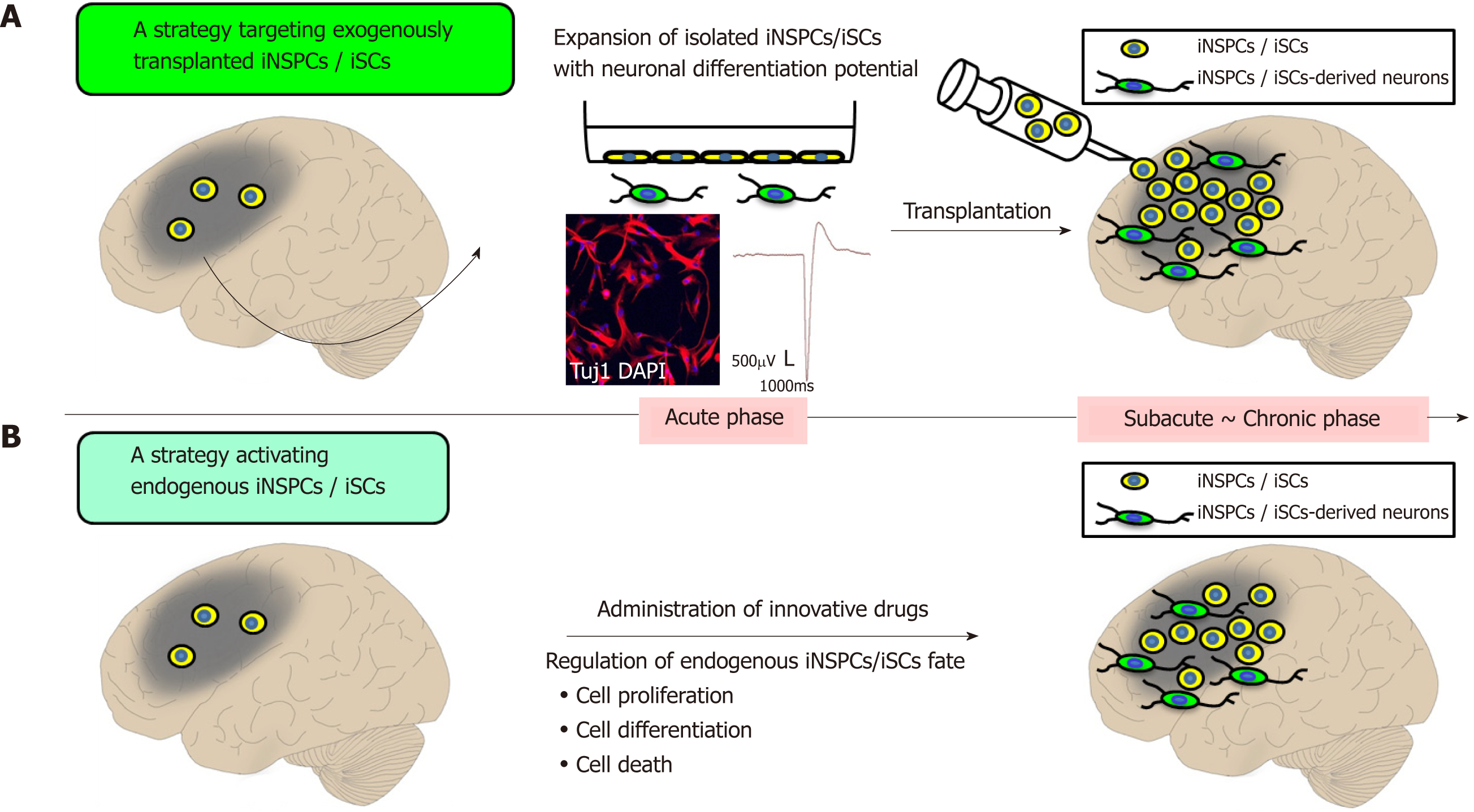
Figure 2 Prospects of regenerative therapy using injury/ischemia-induced neural stem/progenitor cells and injury/ischemia-induced multipotent stem cells.
A: Strategic targeting of exogenously transplanted iNSPCs/iSCs. iNSPCs/iSCs exhibit high proliferative activity and differentiate into electrophysiologically-functional neurons in vitro. Thus, it is expected that transplanted iNSPCs/iSCs can differentiate into neuronal cells in vivo, thereby promoting central nervous system repair; B: A strategy for activating endogenous iNSPCs/iSCs. Administration of bioactive molecules has the potential to promote neural repair by regulating cell proliferation, cell differentiation, and cell death of endogenous iNSPCs/iSCs. iSCs: Injury/ischemia-induced multipotent stem cells; iNSPCs: Injury/ischemia-induced neural stem/progenitor cells.
- Citation: Nakagomi T, Takagi T, Beppu M, Yoshimura S, Matsuyama T. Neural regeneration by regionally induced stem cells within post-stroke brains: Novel therapy perspectives for stroke patients. World J Stem Cells 2019; 11(8): 452-463
- URL: https://www.wjgnet.com/1948-0210/full/v11/i8/452.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i8.452