Copyright
©The Author(s) 2018.
World J Stem Cells. Sep 26, 2018; 10(9): 119-133
Published online Sep 26, 2018. doi: 10.4252/wjsc.v10.i9.119
Published online Sep 26, 2018. doi: 10.4252/wjsc.v10.i9.119
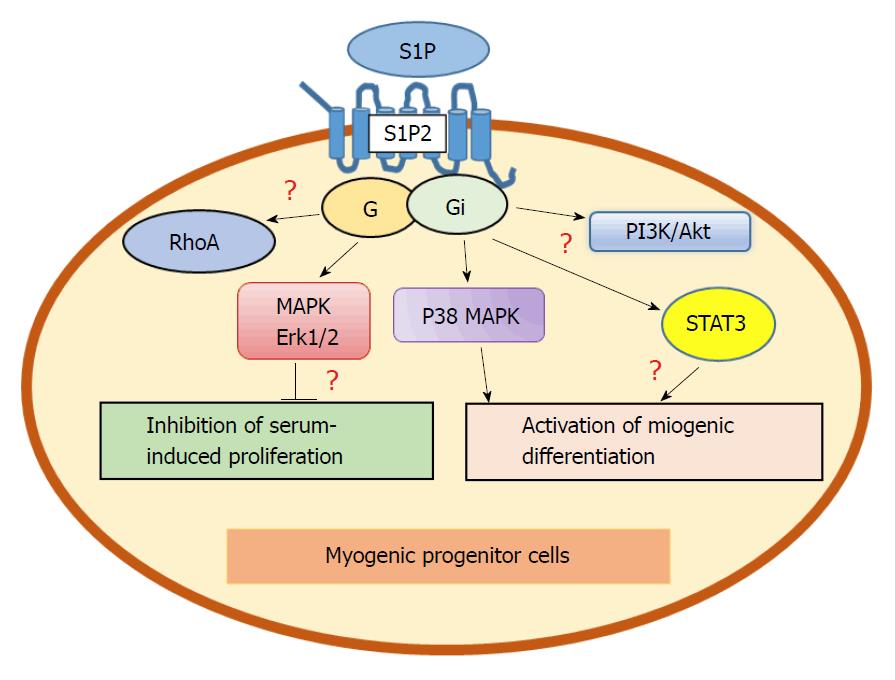
Figure 1 Diversion of myogenic stimulation at the S1P2 receptor level (hypothetical scheme).
Activation of S1P2 receptor signaling results in dual effects in muscle progenitor cells: Erk1/2 mediates inhibition of serum-induced proliferation, while p38MAPK[78,79], phosphatidylinositol 3-kinase (PI3K)[72] and activator of transcription 3 (STAT3)-dependent pathways[82] are required for S1P-trigerred activation of myogenic differentiation. The role of RhoA signaling is unclear. Question marks indicate unclear signaling regulation. S1P: Sphingosine-1-phosphate.
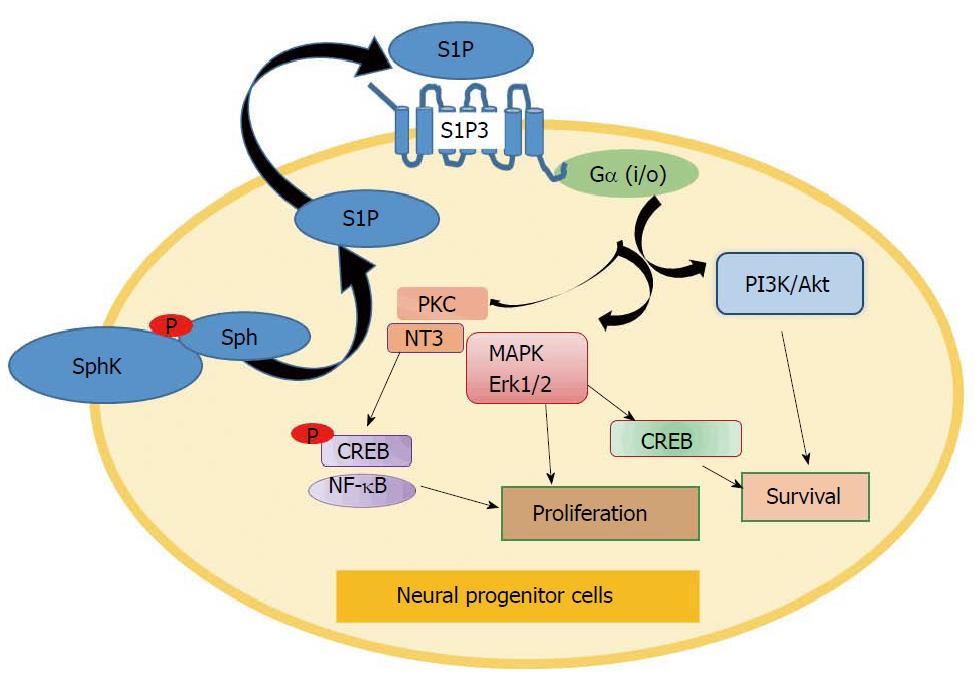
Figure 2 Sphingosine kinase/sphingosine-1-phosphate signaling axis in neural stem/progenitor cells (hypothetical scheme).
Activation of S1P3 receptor signaling and activation of neural cell progenitor differentiation are mediated by various downstream effectors including PKC, PI3K/Akt, MAPK/Erk1/2, NT3, and CREB/NF-κB TFs. SphK: Sphingosine kinase; S1P: Sphingosine-1-phosphate; CREB: cAMP-response element binding protein; NT3: Neurotrophin 3; TF: Transcription factor.
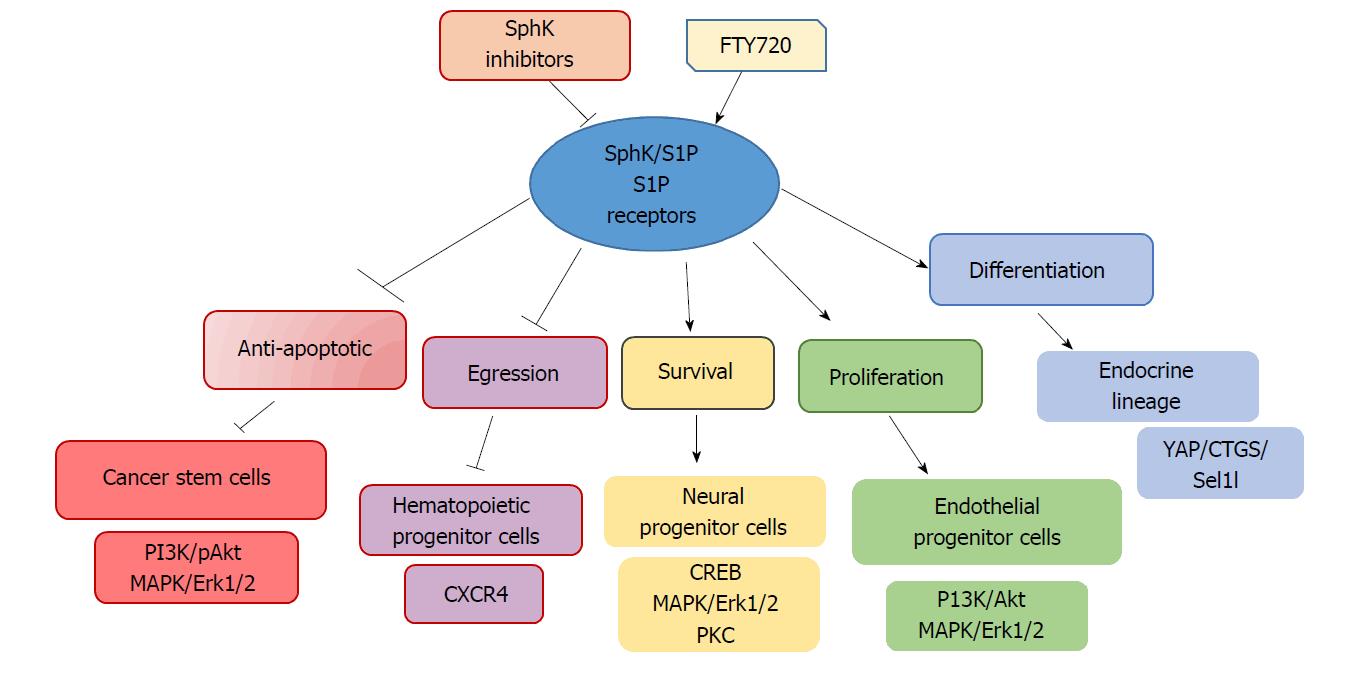
Figure 3 Differentiating effects of sphingosine kinase/sphingosine-1-phosphate inhibition and S1P receptor activation on downstream signaling pathways in various stem/progenitor cells.
SphK: Sphingosine kinase; S1P: Sphingosine-1-phosphate; CREB: cAMP-response element binding protein; YAP: YES-associated protein.
- Citation: Ng ML, Yarla NS, Menschikowski M, Sukocheva OA. Regulatory role of sphingosine kinase and sphingosine-1-phosphate receptor signaling in progenitor/stem cells. World J Stem Cells 2018; 10(9): 119-133
- URL: https://www.wjgnet.com/1948-0210/full/v10/i9/119.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v10.i9.119