修回日期: 2025-06-23
接受日期: 2025-07-17
在线出版日期: 2025-08-28
肠道微生物组与心血管疾病的关联性日益受到关注. 肠道微生物组可通过调节体内的代谢产物影响人体的免疫炎症水平. 肠道菌群失调可对动脉粥样硬化、高血压、心力衰竭等心血管疾病的病理过程产生负面影响. 本文重点阐述肠道微生物组影响心血管疾病的机制, 为该领域临床和转化研究提供参考思路.
核心提要: 肠道微生物组及其代谢产物在动脉粥样硬化、高血压、心力衰竭等心血管疾病的病理过程中发挥重要作用. 靶向肠道微生物组干预有可能改善心血管疾病的发生发展.
引文著录: 田萃红, 谭学瑞. 肠道微生物组与心血管疾病: 证据、机制、挑战与未来策略. 世界华人消化杂志 2025; 33(8): 631-639
Revised: June 23, 2025
Accepted: July 17, 2025
Published online: August 28, 2025
The role of the gut microbiome in cardiovascular diseases has attracted increasing attention. The gut microbiome affects the level of immune inflammation by regulating the metabolites in the body. Gut microbiota dysbiosis has a negative impact on the pathological process of cardiovascular diseases such as atherosclerosis, hypertension, and heart failure. This paper focuses on the mechanism by which the gut microbiome affects cardiovascular diseases, with an aim to provide reference for clinical and translational research in this field.
- Citation: Tian CH, Tan XR. Gut microbiome and cardiovascular disease: Evidence, mechanism, challenge, and future strategies. Shijie Huaren Xiaohua Zazhi 2025; 33(8): 631-639
- URL: https://www.wjgnet.com/1009-3079/full/v33/i8/631.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v33.i8.631
心血管疾病(cardiovascular diseases, CVD)的传统危险因素包括性别、年龄、吸烟、高血压、脂代谢紊乱、糖代谢异常和环境等, 但上述危险因素尚不能完全解释CVD的发病风险[1-3]. 自2011年肠道微生物组(gut microbiome, GM)代谢产物氧化三甲胺(trimethylamine-N-oxide, TMAO)与动脉粥样硬化(atherosclerosis, AS)的关联首次报道以来[4,5], 肠道菌群失调(gut microbiota dysbiosis, GMD)与CVD的关系日益受到重视[6].
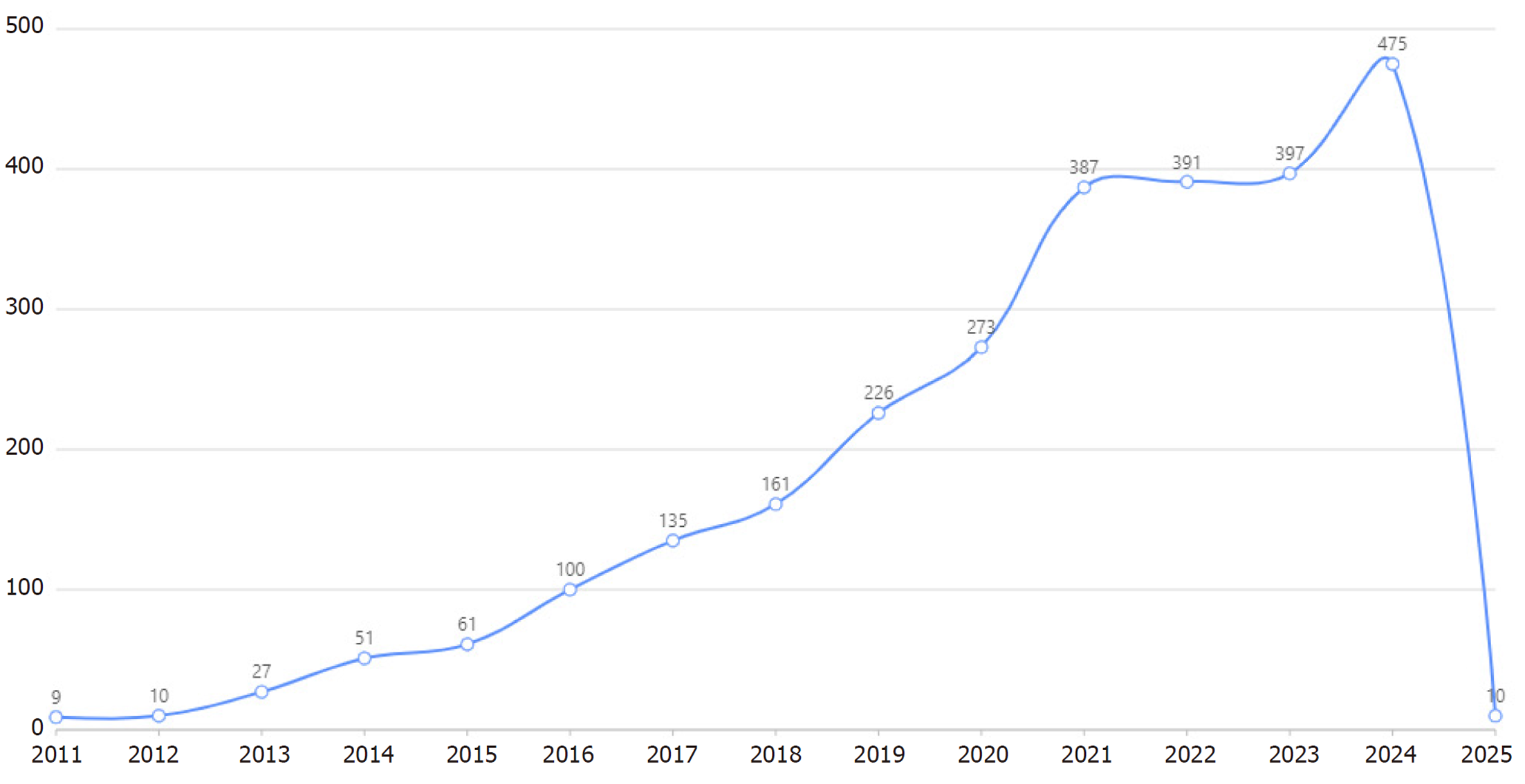
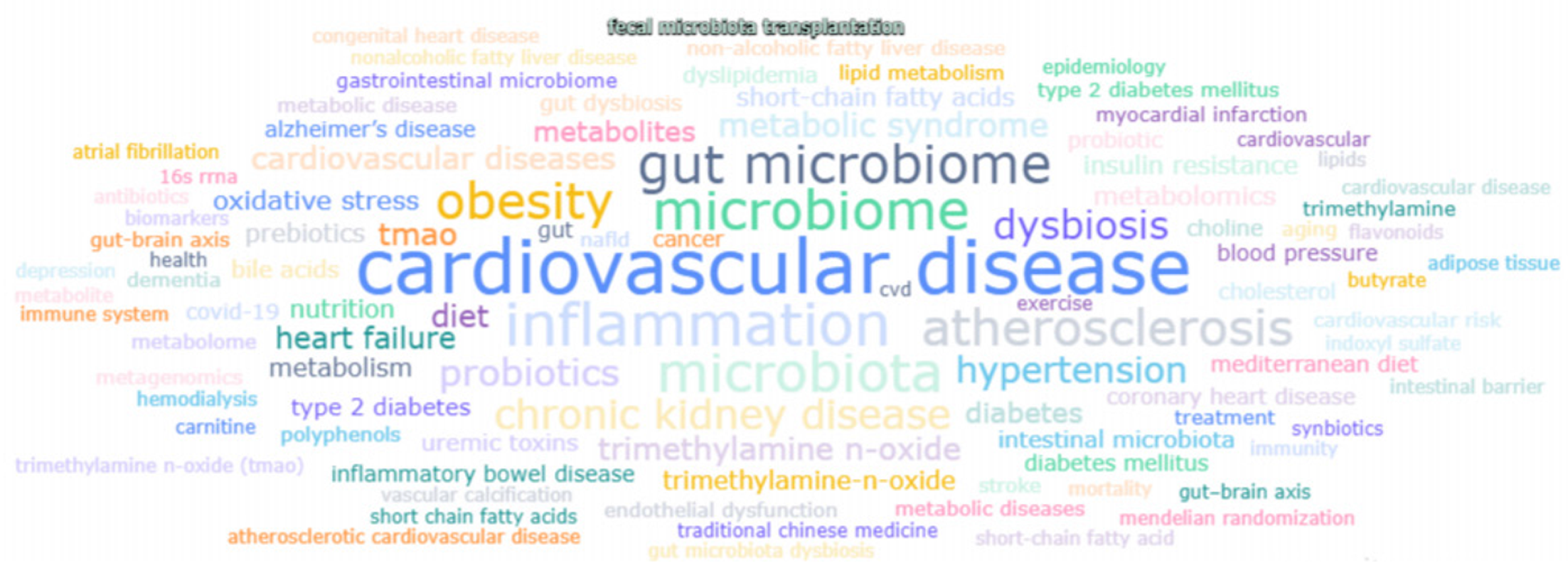
采用文献计量学方法, 以PubMed文献数据库为基础, 使用赛特新思数据分析平台, 以gut microbiota/intestinal flora/intestinal microbiome/gastrointestinal microbiota/gut flora/gut microbiome和cardiovascular disease/heart disease/circulatory system disorder/cardiac illness/heart condition为关键词进行文献挖掘. 自2011-01/2025-04的文献量为2713篇, 平均年发文量181篇. 2024达到475篇的年发文量顶峰, 2013增长率最快为170%, 提示该领域的研究处于快速上升阶段(图1). 热点词频分析发现, 出现频次居前5的关键词分别是: Cardiovascular disease, inflammation, microbiome, microbiota和gut microbiome(图2).
GM的代谢功能与宿主产生交互作用. 脂质代谢紊乱是AS的主要风险因素[7], AS是心脑血管疾病的主要病理生理基础. GM通过代谢、免疫、神经内分泌等多种途径调控心血管稳态[8-10]. 本文从病理生理机制、临床研究的重要证据及转化医学成果等对该领域方向的新进展进行评述.
GM携带300万以上个基因, 相当于人类基因组的150倍, 被称为人类"第二基因组"[11,12]. GM与人体代谢功能交互影响. GM将膳食纤维发酵成乙酸、丙酸、丁酸等短链脂肪酸(Short-chain fatty acids, SCFAs); SCFAs可调节人体能量代谢与免疫平衡[13-15]. 肠道拟杆菌门(Bacteroidetes)和厚壁菌门(Firmicutes)在膳食纤维的发酵上具有协同作用, 是GM代谢的核心[16,17]; 拟杆菌属(Bacteroides)有260多种糖苷水解酶, 可降解木聚糖、果胶等复杂多糖[18]; 瘤胃球菌属(Ruminococcus)通过其特有的纤维小体降解纤维素类物质[19].
GM平均每天产生SCFAs 100-200 mmoL, 其中乙酸占60%, 丙酸占25%, 丁酸占15%[20]. SCFAs参与肝肠代谢. 乙酸是肝脏合成脂质的底物, 并可激活腺苷酸活化蛋白激酶促进脂肪氧化; 丙酸经门静脉进入肝脏, 抑制脂肪酸合成酶表达, 进而抑制胆固醇合成; 丁酸除为结肠上皮供能外, 还可激活过氧化物酶体增殖物激活受体γ保护肠道屏障, 同时还可通过激活G蛋白偶联受体41/43抑制组蛋白去乙酰化酶表达, 促进调节性T细胞分化, 抑制炎症反应[21,22].
GM在AS、高血压以及调节人血小板功能中发挥重要作用[23]. 某些GM代谢产物是多种慢性病发生的关键介质. TMAO和苯乙酰谷氨酰胺(phenylacetylglutamine, PAGln)是典型的菌群-人体共代谢物, 在AS的发生发展中具有独特的病理作用[24-26]. 近年发现组氨酸生成的一种代谢产物-丙酸咪唑(imidazole propionate, ImP)也与CVD的发生密切相关.
TMAO代谢及其致AS的病理作用: 膳食中所含的胆碱、磷脂酰胆碱和左旋肉碱等前体物质是TMAO的主要来源. 微生物中的胆碱代谢关键酶胆碱利用蛋白C(choline utilization protein C, CutC)主要存在于GM中, 如梭菌属(Clostridium)、埃希氏菌属(Escherichia)等; 胆碱利用蛋白D(choline utilization protein D, CutD)是CutC的激活蛋白, CutD通过水解ATP提供能量, 结合CutC并诱导其构象变化, 增强其催化活性, 并促进CutC与底物胆碱结合; 肠道中厌氧菌属(Anaerococcus)、埃希氏菌属(Escherichia)通过酶复合体(CutC/D)将胆碱转化成三甲胺, 三甲胺随后由门静脉进入肝脏, 在黄素单加氧酶3催化下进一步被氧化为TMAO.
TMAO与CVD病死率相关[27]. 大规模临床研究显示, 血浆TMAO水平升高与主要不良心血管事件发生风险增加有关[28,29]. 循环TMAO升高的2型糖尿病患者血清肌酐水平加倍, 且进展为终末期肾病和发生死亡的风险较高, 因此, TMAO是2型糖尿病患者肾功能进展和死亡的潜在生物标志物[30]. 此外, 临床队列研究显示, 血浆TMAO水平升高是心肌梗死和脑卒中的独立风险因子[31-33].
TMAO在血浆中的半衰期为8-12 h. TMAO可激活NOD样受体蛋白3、促进泡沫细胞生成、抑制胆固醇逆向转运, 从而加速AS的发生发展[34]. 该过程涉及: (1)诱导炎症激活: TMAO激活Toll样受体4(Toll-like receptor 4, TLR4)/髓样分化因子88(myeloid differentiation primary response 88, MyD88)/核因子κ轻链增强子结合蛋白(nuclear factor kappa-light-chain-enhancer of activated B cells, NF-κB)信号途径; 促进巨噬细胞产生白细胞介素-1β; 诱导血管内皮细胞表达粘附分子1, 招募白细胞[35]; (2)促进脂代谢紊乱: 抑制胆固醇7α-羟化酶(cholesterol 7α-hydroxylase, CYP7A1)基因表达, 降低胆汁酸合成, 引起胆固醇蓄积[36]; (3)加快泡沫细胞形成: 抑制ATP结合盒转运体(ATP-binding cassette transporters, ABC转运体)超家族细胞膜跨膜蛋白和ABCA1介导的胆固醇逆向转运, 导致巨噬细胞胆固醇流出率降低, 从而促进泡沫细胞形成[4,37]; (4)促血栓形成: 上调肌醇1,4,5-三磷酸受体, 促进胶原诱导的血小板聚集, 加速血栓形成[38]; 同时活化血小板表面和内皮细胞跨膜糖蛋白-P-选择素外露. P-选择素通过结合白细胞表面配体(如P-选择素糖蛋白配体-1), 促进血小板与白细胞、内皮细胞之间的黏附, 形成"血小板-白细胞聚集体"; 血小板表面P-选择素暴露后, 招募更多白细胞, 释放促炎因子(如肿瘤坏死因子-α、白细胞介素-6), 加剧血栓形成的级联反应.
PAGln代谢及其致AS的病理作用: 苯丙氨酸经肠道梭菌属(Clostridium)所含的苯丙氨酸脱氨酶转化为苯乙酸. 苯乙酸与谷氨酰胺在肝脏内结合生成PAGln. PAGln具有显著的肾上腺素能受体激动剂特性[39], 可增强血小板活化及血栓形成. 它通过激活磷脂酰肌醇3-激酶/蛋白激酶B和丝裂原活化蛋白激酶通路, 促进血小板脱颗粒(释放二磷酸腺苷、血栓素A2)和整合素αⅡbβ3活化, 增强血小板与纤维蛋白原的结合力[40], 进而促进AS形成.
ImP代谢及其致病作用: ImP是一种由多种菌株(如乳杆菌属、链球菌属、梭菌属等)代谢组氨酸而形成的有害产物. 组氨酸在组氨酸氨裂解酶的作用下生成尿刊酸, 尿刊酸在尿刊酸还原酶的作用下生成ImP.
近年发现, ImP与CVD的发生风险增加相关. 研究发现, 冠状动脉狭窄患者的肠道菌群多样性较低, 血浆ImP水平升高, 产ImP菌如狡诈瘤胃球菌(Rumiococcus gnavus)和韦荣球菌属(Veillonella)的丰度增加[41]. 一项多中心队列研究显示, 中国人群ImP水平是瑞典人群的3倍, 且ImP可增加慢性心力衰竭患者合并症的发生率[42]. 一项临床试验发现, 慢性心力衰竭患者循环中ImP的水平较对照组增高, 可能与肠道通透性增加导致的系统性炎症改变有关[43].
ImP可通过损害内皮细胞功能促进炎症反应导致CVD的发生. 动物实验发现, ImP剂量依赖性地损害了人内皮细胞的迁移和血管生成特性, 并促进了炎症反应的增加. 长期暴露于ImP会降低动脉损伤后内皮细胞的修复能力. 在载脂蛋白E缺陷小鼠中, ImP增加了AS斑块的大小. 在机制上, ImP通过抑制磷脂酰肌醇3-激酶/蛋白激酶B通路导致叉头框蛋白O1(forkhead box protein O1, FOXO1)转录因子的持续激活来减弱胰岛素受体信号传导. 在ImP处理的小鼠中, 内皮FOXO1的失活增强了血管生成活性, 并保留了颈动脉损伤后内皮细胞的血管修复能力[44].
在对218名动脉粥样硬化性心血管疾病患者和187名健康对照者的粪便进行全基因组关联研究的结果显示, 动脉粥样硬化性心血管疾病患者的GM因肠杆菌和链球菌的丰度增加而偏离健康状态[45]. 西班牙一项横断面研究招募了180名年龄在45-74岁之间的受试者, 探索了GM与早期血管老化之间的关系. 发现与对照组相比, 早期血管老化组的胆汁菌属(Bilophila)、粪杆菌UBA1819(Faecalibacterium sp.UBA1819)和福塞亚菌属(Phocea)丰度增加, 策德莱氏菌属(Cedecea)、乳球菌属(Lactococcus)、假单胞菌属(Pseudomonas)及琥珀酸裂解菌属(Succiniclasticum)丰度减低, 而厚壁菌门(Firmicutes)/拟杆菌门(Bacteriodetes)比率、α多样性和β多样性之间无显著差异[46]. 同样在该西班牙群体中发现, GM组成存在性别差异, 女性具有较高的GM多样性和潜在的保护菌属. 具体而言, 与男性相比, 女性中产SCFAs的多尔菌属(Dorea)、罗斯氏菌属(Roseburia)和阿加莎杆菌属(Agathobacter)更为丰富; 在动脉僵硬受试者中, 布劳特氏菌属(Blautia)在女性中的丰度显著高于男性; 罗斯氏菌属(Roseburia)丰度与男性动脉僵硬度呈负相关, 而双歧杆菌属(Bifidobacterium)和亚多颗粒菌属(Subdoligranulum)丰度与动脉僵硬度呈正相关. 这表明宿主性别决定了同一细菌对动脉僵硬度的不同影响[47].
GMD可能通过如下途径参与AS形成: (1)GMD激活脂多糖(lipopolysaccharide, LPS)/TLR4/NF-κB炎症级联通路: GMD造成肠屏障损伤, 导致LPS入血; LPS的脂质A结构域与TLR4-MD2复合物结合, 激活TLR4/NF-κB信号通路, 诱发内皮细胞炎性反应; 同时激活MyD88促进NF-κB核转位, 进而诱导内皮细胞表达血管黏附分子-1; 此外, GMD与LPS协同激活NOD样受体蛋白3炎症小体, 通过半胱天冬酶-1介导白细胞介素-1β的成熟与释放[48,49]; (2)胆汁酸代谢紊乱: GMD抑制法尼醇X受体信号, 导致CYP7A1活性受抑制, 降低其启动子区组蛋白H3第27位赖氨酸乙酰化修饰水平, 减少初级胆汁酸合成, 阻碍胆固醇逆向转运, 降低胆固醇排泄; 上调次级胆汁酸石胆酸, 激活G蛋白偶联胆汁酸受体5, 活化转化生长因子β/抗果蝇decapentaplegic蛋白同源物3/基质金属蛋白酶2等通路, 促进血管平滑肌细胞迁徙[50,51]; (3)表观遗传重编程: 丁酸缺乏导致内皮细胞组蛋白H3的第9位赖氨酸乙酰化水平下降, 进而抑制Kruppel样因子4转录, 上调miR-34a转录, 加快细胞衰老; 此外, 菌群来源的miR-223-3p通过胞外囊泡转运至斑块巨噬细胞内, 并抑制ABCA1表达[52-54].
长期高盐摄入会导致GMD, 并导致GM相关代谢物表达的显著变化. 在这些代谢产物中, SCFAs、TMAO、氨基酸、胆汁酸和LPS是微生物-宿主相互作用的重要介质, 可能通过炎症、免疫、血管和神经等途径促进盐敏感性高血压的发生发展[55]. 一项随机对照试验表明, 补充膳食纤维可调节肠道菌群组成, 降低血压、减少抗高血压药物的使用[56]. GM与高血压的关系存在性别差异[57]. 一项横断面研究显示, 女性中高血压组与对照组的β多样性和GM组成存在显著差异, 而男性中没有观察到这种差异. 具体而言, 在高血压女性中, 迟钝瘤胃球菌(Ruminococcus gnavus)、博氏梭菌(Clostridium bolteae)和卵形拟杆菌(Bacteroides ovatus)的含量明显高于对照组, 而正常血压女性中, 产甲酸多拉菌(Dorea formicigenerans)的含量更高. 此外, 血浆总SCFAs和丙酸是女性而非男性血压水平的独立预测因素. 这表明[58], 在评估GM对高血压的发展和治疗时, 性别差异可能是一个重要的考虑因素.
由金霉素链霉菌(S. aureofaciens)Tü117产生的α-脂霉素在高盐饮食小鼠和高血压患者的血清中上调. α-脂霉素通过瞬时受体电位香草酸4介导的一氧化氮和内皮衍生超极化因子途径, 损害小鼠血管舒张功能. 植物乳杆菌(L. plantarum)CCFM639可能通过抑制金霉素链霉菌(S. aureofaciens)Tü117在小鼠体内的增殖降低血压. 补充植物乳杆菌(L. plantarum)CCFM639可降低新诊断为高血压前期或高血压1级、且未服用抗高血压药物的受试者的血压. 这提示靶向GM可作为高血压的新干预措施[59].
GM代谢产物SCFAs可能通过中枢机制(肠-脑轴)调节血压. SCFAs可刺激肠内分泌细胞释放神经递质和激素, 如5-羟色胺、胆囊收缩素、胰高血糖素样肽1和肽YY. 这些激素与外周神经系统(如迷走神经和脊髓神经)上的受体结合, 将信息传递给大脑. 除神经体液机制外, 免疫细胞如T淋巴细胞和B淋巴细胞也在神经递质到迷走神经的信号传递过程中发挥促进作用. 理解和利用这些机制将有助于开发治疗高血压的新疗法[60].
一项关于PAGln与心力衰竭的临床和基础研究, 纳入了接受冠状动脉造影的2个独立队列(发现队列N = 3256; 欧洲队列N = 829)人群, 发现循环PAGln水平与心力衰竭的存在和严重程度呈剂量反应性相关, 而与传统危险因素和肾功能无关; PAGln及其小鼠对应物苯乙酰甘氨酸使心肌细胞肌节收缩能力减少, B型利钠肽基因表达增加[61]. 肠-心轴可能是该现象发生的关键机制[62]. 这提示调节GM, 特别是PAGln的产生, 可能是心力衰竭的潜在治疗靶点. 基于GM、TMAO和全身炎症与心力衰竭相关性的认识, 有学者提出用益生菌治疗改善心力衰竭, 但未获阳性结果, 证据来源于一项多中心、前瞻性随机开放标签、盲法终点的试验. 该研究将心力衰竭患者随机分为使用益生菌酵母布拉氏酵母菌(Saccharomyces boulardii)、抗生素利福昔明、标准治疗三组, 发现在标准治疗基础上, 使用布拉氏酵母菌(Saccharomyces boulardii)或利福昔明治疗三个月, 心力衰竭患者的左室射血分数、微生物群多样性、TMAO和C反应蛋白无显著差异[63].
GM靶向干预与传统CVD管理方法相结合, 有望降低CVD的发生风险. AS患者的微生态表型特征可作为生物标志物和治疗的潜在靶点, 如致病菌属(巨单胞菌Megamonas、韦洛氏菌Veillonella、链球菌Streptococcus)的丰度增加和抗炎相关菌属(双歧杆菌Bifidobacterium、罗氏菌属Roseburia)的丰度减少. 益生菌是对人体健康有益的活性微生物, 可以改善消化功能, 抑制有害菌的繁殖, 增强免疫力. 益生元是不可消化的膳食纤维, 可以刺激肠道有益细菌的生长和活性. 针对GM的干预措施, 如补充益生菌或益生元和饮食调整, 是恢复微生物平衡和降低CVD风险的有效方法[64].
动物实验研究初步证实了粪菌移植对遗传缺陷导致的AS发病的影响. 该实验比较了AS易感小鼠模型[C1q/TNF相关蛋白9敲除(CTRP9-KO)小鼠]和野生型小鼠的GM组成, 并进行了粪菌移植以确认GM与AS进展之间的关联. 结果显示粪菌移植在很大程度上影响了CTRP9-KO小鼠和野生型小鼠的GM, 所有粪菌移植小鼠都获得了供体小鼠的GM. 与移植前相比, 粪菌移植后的CTRP9-KO小鼠颈动脉粥样硬化病变减少; 将野生型小鼠粪菌移植到CTRP9-KO小鼠中可抑制AS的进展, 而将CTRP9-KO小鼠粪菌移植到野生型小鼠体内促进了AS的进展. 该研究表明[65], 恢复肠微生物稳态可能是AS的一种有效治疗策略. 粪菌移植的作用在慢性根尖周炎诱导AS小鼠模型中得到了进一步验证[66].
粪菌移植在高血压患者中的改善作用也得到了证实. 一项多中心、随机、盲法、安慰剂对照试验将124例高血压患者随机分为了口服粪菌移植胶囊组和安慰剂组, 以评估粪菌移植的安全性和有效性. 研究发现, 粪菌移植组与安慰剂组不良事件的发生率无统计学差异, 二者安全性相当; 粪菌移植1周即可显著降低高血压患者的收缩压(组间差-4.34 mmHg, 95%CI: -8.1至-0.58; P = 0.024). 此外, 该研究还鉴定出14种与血压调控相关的菌种(如迟缓埃格特菌Eggerthella lenta、粪便副拟杆菌Parabacteroides merdae)和8种与血压调控相关的氨基酸代谢物, 包括酪氨酸、谷氨酰胺、天冬氨酸、苯丙氨酸、蛋氨酸、丝氨酸、肌氨酸、天冬酰胺[67].
观察性研究有其自身的缺陷, 其突出的问题是因果关系难以确定[68]. 而现有GM相关研究约75%是基于观察性分析. 未来可采用如下方式加以确定: (1)无菌动物模型[69]: 将人源菌群移植给载脂蛋白E缺陷的无菌鼠, 结合16S rRNA基因测序、全基因组测序或宏基因组学等技术判定细菌种类是否参与斑块形成[70,71]; (2)时序性干预: 在不同病理阶段用抗生素或益生菌干预, 结合正电子发射计算机断层显像动态观察斑块代谢活性变化; (3)孟德尔随机化分析: 利用菌群相关单核苷酸多态性作为工具变量, 研究因果关系[72,73].
GM及其代谢网络的变化在AS、高血压、心力衰竭等CVD的病理过程中发挥着至关重要的作用. 靶向肠道微生物组干预结合传统CVD治疗方法有望进一步改善CVD的发生发展. 然而针对该领域研究因果关系难以确定和临床转化困难等问题仍需进一步探索.
学科分类: 胃肠病学和肝病学
手稿来源地: 广东省
同行评议报告学术质量分类
A级 (优秀): A
B级 (非常好): B, B, B, B
C级 (良好): C, C
D级 (一般): 0
E级 (差): 0
科学编辑: 刘继红 制作编辑:张砚梁
| 1. | Lazzerini PE, Hamilton RM, Boutjdir M. Editorial: Cardioimmunology: Inflammation and Immunity in Cardiovascular Disease. Front Cardiovasc Med. 2019;6:181. [PubMed] [DOI] |
| 2. | Shi J, Cheng Y, Wang C, Liu M, Qu M, Zhou S, Chen L, Li X, Luo J, Luo Y, Luo C, An P. Effects of Celastrol-Enriched Peanuts on Metabolic Health and the Development of Atherosclerosis. Nutrients. 2025;17:1418. [PubMed] [DOI] |
| 3. | Muttiah B, Hanafiah A. Gut Microbiota and Cardiovascular Diseases: Unraveling the Role of Dysbiosis and Microbial Metabolites. Int J Mol Sci. 2025;26:4264. [PubMed] [DOI] |
| 4. | Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57-63. [PubMed] [DOI] |
| 5. | Tang WHW, Li XS, Wu Y, Wang Z, Khaw KT, Wareham NJ, Nieuwdorp M, Boekholdt SM, Hazen SL. Plasma trimethylamine N-oxide (TMAO) levels predict future risk of coronary artery disease in apparently healthy individuals in the EPIC-Norfolk prospective population study. Am Heart J. 2021;236:80-86. [PubMed] [DOI] |
| 6. | Leng X, Wei X, Wang J, Yao X, Zhang M, Sun D, Liang J, Chi L, Cheng Y. Impacts of intestinal microbiota metabolite trimethylamine N-oxide on cardiovascular disease: a bibliometric analysis. Front Microbiol. 2024;15:1491731. [PubMed] [DOI] |
| 7. | Björnson E, Adiels M, Taskinen MR, Burgess S, Rawshani A, Borén J, Packard CJ. Triglyceride-rich lipoprotein remnants, low-density lipoproteins, and risk of coronary heart disease: a UK Biobank study. Eur Heart J. 2023;44:4186-4195. [PubMed] [DOI] |
| 8. | Ke Y, Li D, Zhao M, Liu C, Liu J, Zeng A, Shi X, Cheng S, Pan B, Zheng L, Hong H. Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic Biol Med. 2018;116:88-100. [PubMed] [DOI] |
| 9. | Piskol F, Neubauer K, Eggers M, Bode LM, Jasper J, Slusarenko A, Reijerse E, Lubitz W, Jahn D, Moser J. Two-component carnitine monooxygenase from Escherichia coli: functional characterization, inhibition and mutagenesis of the molecular interface. Biosci Rep. 2022;42:BSR20221102. [PubMed] [DOI] |
| 10. | Singh A, Kishore PS, Khan S. From Microbes to Myocardium: A Comprehensive Review of the Impact of the Gut-Brain Axis on Cardiovascular Disease. Cureus. 2024;16:e70877. [PubMed] [DOI] |
| 11. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [PubMed] [DOI] |
| 12. | Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, Arumugam M, Kultima JR, Prifti E, Nielsen T, Juncker AS, Manichanh C, Chen B, Zhang W, Levenez F, Wang J, Xu X, Xiao L, Liang S, Zhang D, Zhang Z, Chen W, Zhao H, Al-Aama JY, Edris S, Yang H, Wang J, Hansen T, Nielsen HB, Brunak S, Kristiansen K, Guarner F, Pedersen O, Doré J, Ehrlich SD; MetaHIT Consortium, Bork P, Wang J; MetaHIT Consortium. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014;32:834-841. [PubMed] [DOI] |
| 13. | Patloka O, Komprda T, Franke G. Review of the Relationships Between Human Gut Microbiome, Diet, and Obesity. Nutrients. 2024;16:3996. [PubMed] [DOI] |
| 14. | Yoon JH, Do JS, Velankanni P, Lee CG, Kwon HK. Gut Microbial Metabolites on Host Immune Responses in Health and Disease. Immune Netw. 2023;23:e6. [PubMed] [DOI] |
| 15. | Liu Y, Zhong W, Li X, Shen F, Ma X, Yang Q, Hong S, Sun Y. Diets, Gut Microbiota and Metabolites. Phenomics. 2023;3:268-284. [PubMed] [DOI] |
| 16. | Liu G, Sun H, Liu C, Bai L, Yang L, Jiang W, Gao S. Impact of different dietary fibre sources on production performance, bacterial composition and metabolites in the caecal contents of rabbits. J Anim Physiol Anim Nutr (Berl). 2023;107:1279-1293. [PubMed] [DOI] |
| 17. | Lin D, Peters BA, Friedlander C, Freiman HJ, Goedert JJ, Sinha R, Miller G, Bernstein MA, Hayes RB, Ahn J. Association of dietary fibre intake and gut microbiota in adults. Br J Nutr. 2018;120:1014-1022. [PubMed] [DOI] |
| 18. | Shin YJ, Woo SH, Jeong HM, Kim JS, Ko DS, Jeong DW, Lee JH, Shim JH. Characterization of novel α-galactosidase in glycohydrolase family 97 from Bacteroides thetaiotaomicron and its immobilization for industrial application. Int J Biol Macromol. 2020;152:727-734. [PubMed] [DOI] |
| 19. | Venditto I, Luis AS, Rydahl M, Schückel J, Fernandes VO, Vidal-Melgosa S, Bule P, Goyal A, Pires VM, Dourado CG, Ferreira LM, Coutinho PM, Henrissat B, Knox JP, Baslé A, Najmudin S, Gilbert HJ, Willats WG, Fontes CM. Complexity of the Ruminococcus flavefaciens cellulosome reflects an expansion in glycan recognition. Proc Natl Acad Sci USA. 2016;113:7136-7141. [PubMed] [DOI] |
| 20. | Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, Nauta A, Scott K, Stahl B, van Harsselaar J, van Tol R, Vaughan EE, Verbeke K. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11:411-455. [PubMed] [DOI] |
| 21. | Gao J, Mang Q, Sun Y, Xu G. Short-Chain Fatty Acids (SCFAs) Modulate the Hepatic Glucose and Lipid Metabolism of Coilia nasus via the FFAR/AMPK Signaling Pathway In Vitro. Int J Mol Sci. 2025;26:3654. [PubMed] [DOI] |
| 22. | Sharma T, Ranawat P, Garg A, Rastogi P, Kaushal N. Short-chain fatty acids as a novel intervention for high-fat diet-induced metabolic syndrome. Mol Cell Biochem. 2025;480:3169-3184. [PubMed] [DOI] |
| 23. | Duttaroy AK. Role of Gut Microbiota and Their Metabolites on Atherosclerosis, Hypertension and Human Blood Platelet Function: A Review. Nutrients. 2021;13:144. [PubMed] [DOI] |
| 24. | Deng ZH, Li X, Liu L, Zeng HM, Chen BF, Peng J. Role of gut microbiota and Helicobacter pylori in inflammatory bowel disease through immune-mediated synergistic actions. World J Gastroenterol. 2024;30:5097-5103. [PubMed] [DOI] |
| 25. | Wang Z, Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 2018;9:416-431. [PubMed] [DOI] |
| 26. | Witkowski M, Weeks TL, Hazen SL. Gut Microbiota and Cardiovascular Disease. Circ Res. 2020;127:553-570. [PubMed] [DOI] |
| 27. | Konieczny RA, Żurawska-Płaksej E, Kaaz K, Czapor-Irzabek H, Bombała W, Mysiak A, Kuliczkowski W. Citrulline and long-term mortality in patients with cardiovascular disease. Adv Clin Exp Med. 2022;31:1121-1128. [PubMed] [DOI] |
| 28. | Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Räber L, Windecker S, Rodondi N, Nanchen D, Muller O, Miranda MX, Matter CM, Wu Y, Li L, Wang Z, Alamri HS, Gogonea V, Chung YM, Tang WH, Hazen SL, Lüscher TF. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38:814-824. [PubMed] [DOI] |
| 29. | Croyal M, Saulnier PJ, Aguesse A, Gand E, Ragot S, Roussel R, Halimi JM, Ducrocq G, Cariou B, Montaigne D, Wargny M, Krempf M, Hadjadj S. Plasma Trimethylamine N-Oxide and Risk of Cardiovascular Events in Patients With Type 2 Diabetes. J Clin Endocrinol Metab. 2020;105:dgaa188. [PubMed] [DOI] |
| 30. | Yu PS, Wu PH, Hung WW, Lin MY, Zhen YY, Hung WC, Chang JM, Tsai JR, Chiu YW, Hwang SJ, Tsai YC. Association Between Trimethylamine N-oxide and Adverse Kidney Outcomes and Overall Mortality in Type 2 Diabetes Mellitus. J Clin Endocrinol Metab. 2024;109:2097-2105. [PubMed] [DOI] |
| 31. | Senthong V, Li XS, Hudec T, Coughlin J, Wu Y, Levison B, Wang Z, Hazen SL, Tang WH. Plasma Trimethylamine N-Oxide, a Gut Microbe-Generated Phosphatidylcholine Metabolite, Is Associated With Atherosclerotic Burden. J Am Coll Cardiol. 2016;67:2620-2628. [PubMed] [DOI] |
| 32. | Haghikia A, Li XS, Liman TG, Bledau N, Schmidt D, Zimmermann F, Kränkel N, Widera C, Sonnenschein K, Haghikia A, Weissenborn K, Fraccarollo D, Heimesaat MM, Bauersachs J, Wang Z, Zhu W, Bavendiek U, Hazen SL, Endres M, Landmesser U. Gut Microbiota-Dependent Trimethylamine N-Oxide Predicts Risk of Cardiovascular Events in Patients With Stroke and Is Related to Proinflammatory Monocytes. Arterioscler Thromb Vasc Biol. 2018;38:2225-2235. [PubMed] [DOI] |
| 33. | Qi J, You T, Li J, Pan T, Xiang L, Han Y, Zhu L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med. 2018;22:185-194. [PubMed] [DOI] |
| 34. | Chen ML, Zhu XH, Ran L, Lang HD, Yi L, Mi MT. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J Am Heart Assoc. 2017;6:e006347. [PubMed] [DOI] |
| 35. | Chang SY, Li YT, Zhu HY, He ZX, You Y, Liu YH. Buyang Huanwu Decoction stabilizes atherosclerotic vulnerable plaques by regulating intestinal flora, TLR4-NF-κB-NLRP3 inflammatory pathway and mitophagy. Phytomedicine. 2025;142:156751. [PubMed] [DOI] |
| 36. | Lim T, Lee K, Kim RH, Ryu J, Cha KH, Park SY, Koo SY, Hwang KT. Effects of black raspberry extract on gut microbiota, microbial metabolites, and expressions of the genes involved in cholesterol and bile acid metabolisms in rats fed excessive choline with a high-fat diet. Food Sci Biotechnol. 2023;32:577-587. [PubMed] [DOI] |
| 37. | Yang Y, Karampoor S, Mirzaei R, Borozdkin L, Zhu P. The interplay between microbial metabolites and macrophages in cardiovascular diseases: A comprehensive review. Int Immunopharmacol. 2023;121:110546. [PubMed] [DOI] |
| 38. | Cheng TY, Lee TW, Li SJ, Lee TI, Chen YC, Kao YH, Higa S, Chen PH, Chen YJ. Short-chain fatty acid butyrate against TMAO activating endoplasmic-reticulum stress and PERK/IRE1-axis with reducing atrial arrhythmia. J Adv Res. 2025;73:549-560. [PubMed] [DOI] |
| 39. | He M, Liu A, Shi J, Xu YJ, Liu Y. Multi-Omics Reveals the Effects of Cannabidiol on Gut Microbiota and Metabolic Phenotypes. Cannabis Cannabinoid Res. 2024;9:714-727. [PubMed] [DOI] |
| 40. | Hatamnejad MR, Medzikovic L, Dehghanitafti A, Rahman B, Vadgama A, Eghbali M. Role of Gut Microbial Metabolites in Ischemic and Non-Ischemic Heart Failure. Int J Mol Sci. 2025;26:2242. [PubMed] [DOI] |
| 41. | Trøseid M, Molinaro A, Gelpi M, Vestad B, Kofoed KF, Fuchs A, Køber L, Holm K, Benfield T, Ueland PM, Hov JR, Nielsen SD, Knudsen AD. Gut Microbiota Alterations and Circulating Imidazole Propionate Levels Are Associated With Obstructive Coronary Artery Disease in People With HIV. J Infect Dis. 2024;229:898-907. [PubMed] [DOI] |
| 42. | Hua S, Lv B, Qiu Z, Li Z, Wang Z, Chen Y, Han Y, Tucker KL, Wu H, Jin W. Microbial metabolites in chronic heart failure and its common comorbidities. EMBO Mol Med. 2023;15:e16928. [PubMed] [DOI] |
| 43. | Raju SC, Molinaro A, Awoyemi A, Jørgensen SF, Braadland PR, Nendl A, Seljeflot I, Ueland PM, McCann A, Aukrust P, Vestad B, Mayerhofer C, Broch K, Gullestad L, Lappegård KT, Halvorsen B, Kristiansen K, Hov JR, Trøseid M. Microbial-derived imidazole propionate links the heart failure-associated microbiome alterations to disease severity. Genome Med. 2024;16:27. [PubMed] [DOI] |
| 44. | Nageswaran V, Carreras A, Reinshagen L, Beck KR, Steinfeldt J, Henricsson M, Ramezani Rad P, Peters L, Strässler ET, Lim J, Verhaar BJH, Döring Y, Weber C, König M, Steinhagen-Thiessen E, Demuth I, Kränkel N, Leistner DM, Potente M, Nieuwdorp M, Knaus P, Kuebler WM, Ferrell M, Nemet I, Hazen SL, Landmesser U, Bäckhed F, Haghikia A. Gut Microbial Metabolite Imidazole Propionate Impairs Endothelial Cell Function and Promotes the Development of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2025;45:823-839. [PubMed] [DOI] |
| 45. | Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao H, Zhang D, Su Z, Fang Z, Lan Z, Li J, Xiao L, Li J, Li R, Li X, Li F, Ren H, Huang Y, Peng Y, Li G, Wen B, Dong B, Chen JY, Geng QS, Zhang ZW, Yang H, Wang J, Wang J, Zhang X, Madsen L, Brix S, Ning G, Xu X, Liu X, Hou Y, Jia H, He K, Kristiansen K. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8:845. [PubMed] [DOI] |
| 46. | Salvado R, Santos-Minguez S, Lugones-Sánchez C, Gonzalez-Sánchez S, Tamayo-Morales O, Quesada-Rico JA, Benito R, Rodríguez-Sánchez E, Gómez-Marcos MA, Casado-Vicente V, Guimarães-Cunha P, Hernandez-Rivas JM, Mira A, García-Ortiz L; MIVAS investigators. Gut microbiota and its relationship with early vascular ageing in a Spanish population (MIVAS study). Eur J Clin Invest. 2024;54:e14228. [PubMed] [DOI] |
| 47. | Salvado R, Lugones-Sánchez C, Santos-Minguez S, González-Sánchez S, Quesada JA, Benito R, Rodríguez-Sánchez E, Gómez-Marcos MA, Guimarães-Cunha P, Hernandez-Rivas JM, Mira A, García-Ortiz L; Mivas Investigators. Sex Differences in Gut Microbiota and Their Relation to Arterial Stiffness (MIVAS Study). Nutrients. 2024;17:53. [PubMed] [DOI] |
| 48. | Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131-144. [PubMed] [DOI] |
| 49. | Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373-384. [PubMed] [DOI] |
| 50. | Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517-526. [PubMed] [DOI] |
| 51. | Pols TW, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, Thomas C, Rizzo G, Gioiello A, Adorini L, Pellicciari R, Auwerx J, Schoonjans K. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14:747-757. [PubMed] [DOI] |
| 52. | Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517-526. [PubMed] [DOI] |
| 53. | Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA. 2008;105:13421-13426. [PubMed] [DOI] |
| 54. | Rayner KJ, Suárez Y, Dávalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernández-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570-1573. [PubMed] [DOI] |
| 55. | Mu YF, Gao ZX, Mao ZH, Pan SK, Liu DW, Liu ZS, Wu P. Perspectives on the involvement of the gut microbiota in salt-sensitive hypertension. Hypertens Res. 2024;47:2351-2362. [PubMed] [DOI] |
| 56. | Xue Y, Cui L, Qi J, Ojo O, Du X, Liu Y, Wang X. The effect of dietary fiber (oat bran) supplement on blood pressure in patients with essential hypertension: A randomized controlled trial. Nutr Metab Cardiovasc Dis. 2021;31:2458-2470. [PubMed] [DOI] |
| 57. | Beale AL, Kaye DM, Marques FZ. The role of the gut microbiome in sex differences in arterial pressure. Biol Sex Differ. 2019;10:22. [PubMed] [DOI] |
| 58. | Virwani PD, Qian G, Hsu MSS, Pijarnvanit TKKTS, Cheung CN, Chow YH, Tang LK, Tse YH, Xian JW, Lam SS, Lee CPI, Lo CCW, Liu RKC, Ho TL, Chow BY, Leung KS, Tsang HW, Lo EKK, Tung KTS, Chung SK, Yuen MF, Leung SY, Ip P, Hung IF, Louie JCY, El-Nezami H, Ho JWK, Lau KK. Sex Differences in Association Between Gut Microbiome and Essential Hypertension Based on Ambulatory Blood Pressure Monitoring. Hypertension. 2023;80:1331-1342. [PubMed] [DOI] |
| 59. | Zhou T, Wang Z, Lv X, Guo M, Zhang N, Liu L, Geng L, Shao J, Zhang K, Gao M, Mao A, Zhu Y, Yu F, Feng L, Wang X, Zhai Q, Chen W, Ma X. Targeting gut S. aureofaciens Tü117 serves as a new potential therapeutic intervention for the prevention and treatment of hypertension. Cell Metab. 2025;37:496-513.e11. [PubMed] [DOI] |
| 60. | Welathanthree M, Keating DJ, Macefield VG, Carnevale D, Marques FZ, R Muralitharan R. Cross-talk between microbiota-gut-brain axis and blood pressure regulation. Clin Sci (Lond). 2025;139:431-447. [PubMed] [DOI] |
| 61. | Romano KA, Nemet I, Prasad Saha P, Haghikia A, Li XS, Mohan ML, Lovano B, Castel L, Witkowski M, Buffa JA, Sun Y, Li L, Menge CM, Demuth I, König M, Steinhagen-Thiessen E, DiDonato JA, Deb A, Bäckhed F, Tang WHW, Naga Prasad SV, Landmesser U, Van Wagoner DR, Hazen SL. Gut Microbiota-Generated Phenylacetylglutamine and Heart Failure. Circ Heart Fail. 2023;16:e009972. [PubMed] [DOI] |
| 62. | Abdulrahim AO, Doddapaneni NSP, Salman N, Giridharan A, Thomas J, Sharma K, Abboud E, Rochill K, Shreelakshmi B, Gupta V, Lakkimsetti M, Mowo-Wale A, Ali N. The gut-heart axis: a review of gut microbiota, dysbiosis, and cardiovascular disease development. Ann Med Surg (Lond). 2025;87:177-191. [PubMed] [DOI] |
| 63. | Awoyemi A, Mayerhofer C, Felix AS, Hov JR, Moscavitch SD, Lappegård KT, Hovland A, Halvorsen S, Halvorsen B, Gregersen I, Svardal A, Berge RK, Hansen SH, Götz A, Holm K, Aukrust P, Åkra S, Seljeflot I, Solheim S, Lorenzo A, Gullestad L, Trøseid M, Broch K. Rifaximin or Saccharomyces boulardii in heart failure with reduced ejection fraction: Results from the randomized GutHeart trial. EBioMedicine. 2021;70:103511. [PubMed] [DOI] |
| 64. | Alexandrescu L, Suceveanu AP, Stanigut AM, Tofolean DE, Axelerad AD, Iordache IE, Herlo A, Nelson Twakor A, Nicoara AD, Tocia C, Dumitru A, Dumitru E, Condur LM, Aftenie CF, Tofolean IT. Intestinal Insights: The Gut Microbiome's Role in Atherosclerotic Disease: A Narrative Review. Microorganisms. 2024;12:2341. [PubMed] [DOI] |
| 65. | Kim ES, Yoon BH, Lee SM, Choi M, Kim EH, Lee BW, Kim SY, Pack CG, Sung YH, Baek IJ, Jung CH, Kim TB, Jeong JY, Ha CH. Fecal microbiota transplantation ameliorates atherosclerosis in mice with C1q/TNF-related protein 9 genetic deficiency. Exp Mol Med. 2022;54:103-114. [PubMed] [DOI] |
| 66. | Gan G, Zhang R, Zeng Y, Lu B, Luo Y, Chen S, Lei H, Cai Z, Huang X. Fecal microbiota transplantation validates the importance of gut microbiota in an ApoE(-/-) mouse model of chronic apical periodontitis-induced atherosclerosis. BMC Oral Health. 2024;24:1455. [PubMed] [DOI] |
| 67. | Fan L, Chen J, Zhang Q, Ren J, Chen Y, Yang J, Wang L, Guo Z, Bu P, Zhu B, Zhao Y, Wang Y, Liu X, Wang W, Chen Z, Gao Q, Zheng L, Cai J. Fecal microbiota transplantation for hypertension: an exploratory, multicenter, randomized, blinded, placebo-controlled trial. Microbiome. 2025;13:133. [PubMed] [DOI] |
| 68. | Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. [PubMed] [DOI] |
| 69. | Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14:79-87. [PubMed] [DOI] |
| 70. | Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE. Human genetics shape the gut microbiome. Cell. 2014;159:789-799. [PubMed] [DOI] |
| 71. | Pu Y, Zhou X, Cai H, Lou T, Liu C, Kong M, Sun Z, Wang Y, Zhang R, Zhu Y, Ye L, Zheng Y, Zhu B, Quan Z, Zhao G, Zheng Y. Impact of DNA Extraction Methods on Gut Microbiome Profiles: A Comparative Metagenomic Study. Phenomics. 2025;5:76-90. [PubMed] [DOI] |
| 72. | Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, Mujagic Z, Masclee AAM, Jonkers DMAE, Oosting M, Joosten LAB, Netea MG, Franke L, Zhernakova A, Fu J, Wijmenga C, McCarthy MI. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019;51:600-605. [PubMed] [DOI] |
| 73. | Kurilshikov A, Medina-Gomez C, Bacigalupe R, Radjabzadeh D, Wang J, Demirkan A, Le Roy CI, Raygoza Garay JA, Finnicum CT, Liu X, Zhernakova DV, Bonder MJ, Hansen TH, Frost F, Rühlemann MC, Turpin W, Moon JY, Kim HN, Lüll K, Barkan E, Shah SA, Fornage M, Szopinska-Tokov J, Wallen ZD, Borisevich D, Agreus L, Andreasson A, Bang C, Bedrani L, Bell JT, Bisgaard H, Boehnke M, Boomsma DI, Burk RD, Claringbould A, Croitoru K, Davies GE, van Duijn CM, Duijts L, Falony G, Fu J, van der Graaf A, Hansen T, Homuth G, Hughes DA, Ijzerman RG, Jackson MA, Jaddoe VWV, Joossens M, Jørgensen T, Keszthelyi D, Knight R, Laakso M, Laudes M, Launer LJ, Lieb W, Lusis AJ, Masclee AAM, Moll HA, Mujagic Z, Qibin Q, Rothschild D, Shin H, Sørensen SJ, Steves CJ, Thorsen J, Timpson NJ, Tito RY, Vieira-Silva S, Völker U, Völzke H, Võsa U, Wade KH, Walter S, Watanabe K, Weiss S, Weiss FU, Weissbrod O, Westra HJ, Willemsen G, Payami H, Jonkers DMAE, Arias Vasquez A, de Geus EJC, Meyer KA, Stokholm J, Segal E, Org E, Wijmenga C, Kim HL, Kaplan RC, Spector TD, Uitterlinden AG, Rivadeneira F, Franke A, Lerch MM, Franke L, Sanna S, D'Amato M, Pedersen O, Paterson AD, Kraaij R, Raes J, Zhernakova A. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. 2021;53:156-165. [PubMed] [DOI] |
| 74. | Liu JM, Zhao N, Wang ZH, Lv SW, Li CY, Wang S. In-Taken Labeling and in Vivo Tracing Foodborne Probiotics via DNA-Encapsulated Persistent Luminescence Nanoprobe Assisted Autofluorescence-Free Bioimaging. J Agric Food Chem. 2019;67:514-519. [PubMed] [DOI] |
| 75. | Chapman BC, Moore HB, Overbey DM, Morton AP, Harnke B, Gerich ME, Vogel JD. Fecal microbiota transplant in patients with Clostridium difficile infection: A systematic review. J Trauma Acute Care Surg. 2016;81:756-764. [PubMed] [DOI] |
| 76. | DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen YB, Hohmann EL. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med. 2019;381:2043-2050. [PubMed] [DOI] |
| 77. | Luqman A, Hassan A, Ullah M, Naseem S, Ullah M, Zhang L, Din AU, Ullah K, Ahmad W, Wang G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front Immunol. 2024;15:1321395. [PubMed] [DOI] |
| 78. | Khan MT, Bäckhed F. Development of Next Generation Probiotics for Cardiometabolic Diseases. Phenomics. 2025;5:18-22. [PubMed] [DOI] |
| 79. | Wu H, Forslund S, Wang Z, Zhao G. Human Gut Microbiome Researches Over the Last Decade: Current Challenges and Future Directions. Phenomics. 2025;5:1-7. [PubMed] [DOI] |
| 80. | Datta S, Pasham S, Inavolu S, Boini KM, Koka S. Role of Gut Microbial Metabolites in Cardiovascular Diseases-Current Insights and the Road Ahead. Int J Mol Sci. 2024;25:10208. [PubMed] [DOI] |