修回日期: 2021-06-27
接受日期: 2021-09-15
在线出版日期: 2021-10-28
非甾体类抗炎药(non-steroidal anti-inflammatory drugs, NSAIDs)是一种抗炎镇痛及抗血小板药物, 在全世界被广泛使用. 但随着NSAIDs的长期应用, 其并发症逐渐受到人们的关注. 以往在临床上关注较多的是其导致的上消化道并发症, 但随着内窥镜如胶囊内镜和双气囊小肠镜等的普及, 人们发现NSAIDs引起的小肠损伤也并不少见. 虽然大部分患者并无明显症状, 但仍有部分患者出现了明显的症状或复杂的溃疡, 需要进行治疗干预. 然而, 针对NSAIDs相关性肠病的药物尚未被研发, 因此, 进一步研究NSAIDs相关肠病的发病机制并制定合适的防治策略是有必要的. 本文对NSAIDs所致小肠损伤的流行病学、临床表现、诊断、危险因素、发病机制和治疗进行了归纳总结, 为NSAIDs的使用及其并发症的防治提供参考.
核心提要: 非甾体类抗炎药(non-steroidal anti-inflammatory drugs, NSAIDs)相关小肠损伤在内窥镜下发现并不少见, 部分患者出现严重的并发症. 因此, 应当充分对NSAIDs相关小肠损伤的机制以及防治进行研究, 以降低NSAIDs引起的小肠损伤.
引文著录: 罗洋, 朱兰平, 雷月, 赵经文, 王邦茂, 陈鑫. 非甾体类抗炎药相关小肠损伤的研究进展. 世界华人消化杂志 2021; 29(20): 1191-1200
Revised: June 27, 2021
Accepted: September 15, 2021
Published online: October 28, 2021
Non-steroidal anti-inflammatory drugs (NSAIDs) are used widely around the world because of their anti-inflammatory, analgesic, and antiplatelet activity. However, long-term application of NSAIDs can lead to complications. Previously, the clinical attention was dedicated to the NSAID-induced upper gastrointestinal complications. Recently, the detection rate of small intestinal damage related to NSAIDs has increased due to the wide use of endoscopes such as capsule endoscopy and double-balloon colonoscopy. Although the majority of patients have no significant symptoms, there are still a small percentage of patients who develop obvious symptoms or complicated ulcers that require therapeutic intervention. Despite significant advances in our understanding of NSAIDs, the treatment modality and regimen for NSAID-induced small intestinal damage have remained relatively unclear. This article will provide a comprehensive overview of NSAID-induced small intestinal damage with regard to the epidemiology, clinical manifestations, diagnosis, risk factors, pathogenesis, and treatment, in order to provide informative evidence for clinical practice.
- Citation: Luo Y, Zhu LP, Lei Y, Zhao JW, Wang BM, Chen X. Research progress of non-steroidal anti-inflammatory drug-induced small intestinal injury. Shijie Huaren Xiaohua Zazhi 2021; 29(20): 1191-1200
- URL: https://www.wjgnet.com/1009-3079/full/v29/i20/1191.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v29.i20.1191
自从1899年阿司匹林被合成以来, 非甾体类抗炎药(non-steroidal anti-inflammatory drugs, NSAIDs)成为世界上使用最广泛的解热、镇痛和抗炎药之一, 一直在临床中被广泛运用[1]. 由于阿司匹林的抗血小板特性, 使得心血管疾病的死亡风险大大降低[2]. 近年来, 研究发现阿司匹林也对某些癌症具有防治作用, 包括乳腺癌[3]、结直肠癌[4]等. 虽然NSAIDs给患者带来了很多益处, 但它们也可以引起许多并发症, 其中以胃肠道并发症最常见. NSAIDs可引起严重的胃肠道损伤, 如出血、穿孔和溃疡等[5], 这往往限制了这些药物的使用. 一直以来, 人们对NSAIDs引起的十二指肠远端损伤的关注较少, 但随着视频胶囊内窥镜检查(video capsule endoscope, VCE)[6]和气囊辅助内窥镜检查(double-balloon enterosocope, DBE)[7]的引入, 人们观察到NSAIDs也可对小肠造成损伤[8], 而且其死亡率高于NSAIDs所引起的上消化道损伤[9]. 本文就NSAIDs所致小肠损伤的流行病学、临床表现、诊断、危险因素、发病机制和治疗作一综述, 为NSAIDs的使用及其并发症的防治提供参考.
NSAIDs很容易被人们获得, 并且在没有规范指导的情况下被随意使用. 据估计, 在全世界范围内, 每天大约有3000万人在服用NSAIDs[10]. 2010年美国全国健康访谈调查(National Health Interview Survey, NHIS)显示, 约有4300万成年人(19.0%)每周至少服用三次阿司匹林, 且服用时间超过3个月. 而超过2900万成年人(12.1%)长期服用NSAIDs. 与2005年相比, 阿司匹林的使用人数增加了57%, NSAIDs的使用人数增加了41%[1]. 除此之外, 通过胶囊内窥镜观察到在长期服用NSAIDs的患者中, 有50%-70%的患者出现了小肠损伤[11], 这比NSAIDs引起的胃损伤更为常见[12], 这些患者有时甚至会引发出血和穿孔等更严重的并发症[13] . 但目前人们在临床工作中对NSAIDs相关小肠损伤重视度仍不够, 由于在长期服用NSAIDs的患者中, 60%-70%的患者的小肠损伤都处于亚临床阶段, 无明显的症状, 当出现了严重并发症后才会被识别[14].
在内窥镜引入之前, NSAIDs相关肠病是通过小肠通透性和炎症的测定诊断的. 51Cr标记的乙二胺四乙酸(ethylene diamine tetraacetic acid, EDTA)对小肠的通透性的测定相对特异, 是诊断NSAIDs相关肠病的最常用的方法[15,16]. 111铟(In)标记白细胞进行闪烁显像可以用于检测小肠炎症[17]. 此外, 钙卫蛋白可用作小肠的炎症标记物, 通过粪便钙卫蛋白定量, 可以评估NSAIDs引起的小肠损伤[18].
VCE和DBE能够使小肠损伤的定位更精确. 与DBE相比, VCE对小肠出血诊断率更高, 可以更好地评估NSAIDs引起的小肠损伤[19]. 因此, 这些诊断方法可以更好地发现NSAIDs引起的小肠损伤.
NSAIDs相关肠病发生率很高, 但大多数患者并无典型临床表现[20]. 60%-70%表现出非特异性症状, 如缺铁性贫血、低白蛋白血症、维生素B12吸收不良、腹泻和急性腹痛.严重者可能出现大出血、狭窄和穿孔等并发症[21].
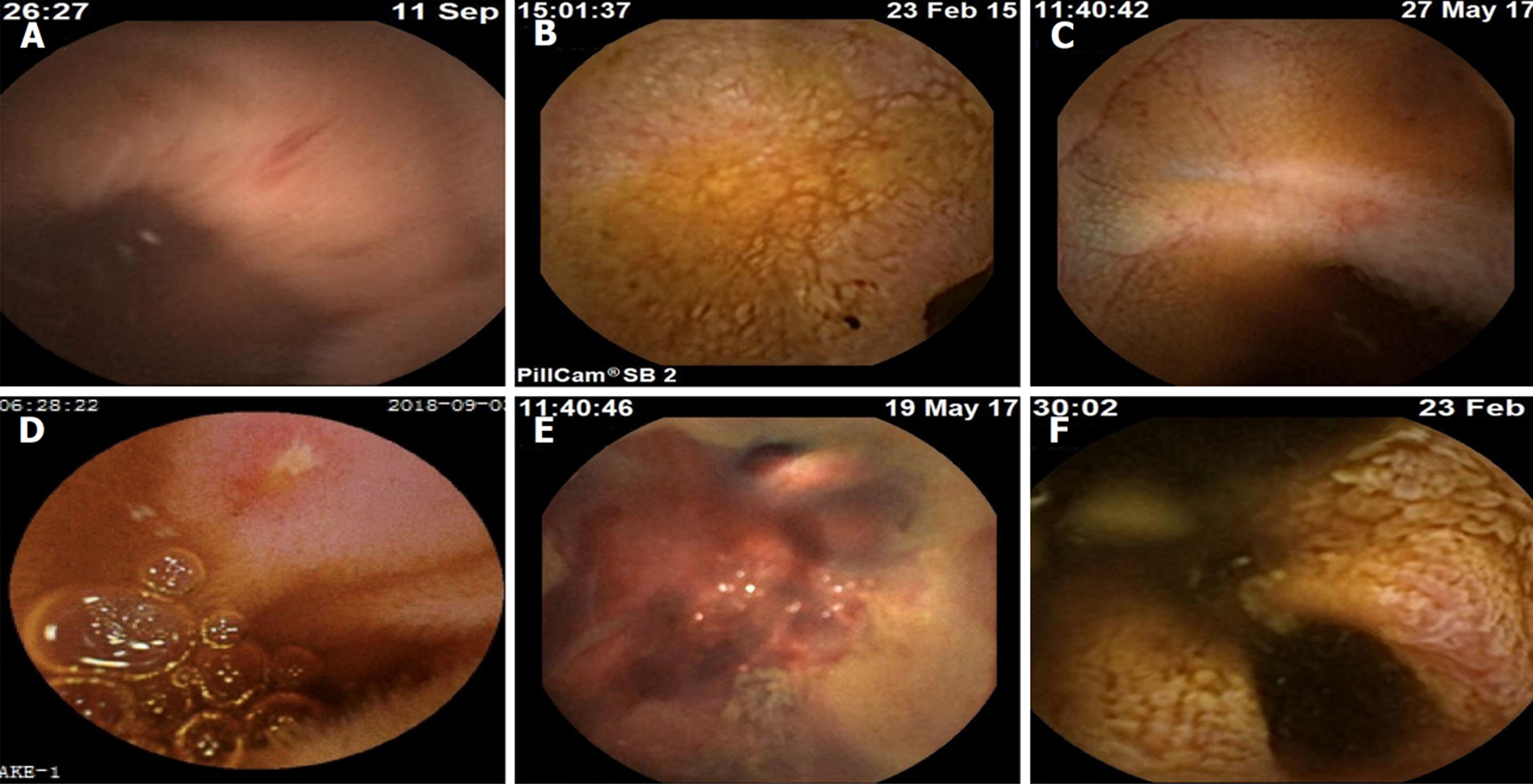
NSAIDs引起的小肠损伤, 即使程度较轻, 也会导致持续性出血和缺铁性贫血. 研究发现47%的小肠溃疡患者, 每天有2-10 mL的失血量[21-23]. 临床上, 明显的出血相对罕见, 多由溃疡和糜烂引起(图1).
危险因素评估对管理NSAIDs引起的小肠损伤是非常重要的, 与NSAIDs引起上消化道损伤相比, 对NSAIDs所致小肠损伤的危险因素的研究有限, 以下是对相关研究的归纳.
质子泵抑制剂(proton pump inhibitors, PPIs)和H2受体拮抗剂, 可明显减少NSAIDs导致的胃和十二指肠的溃疡和出血. 然而, 研究表明[24-26], 这些药物对小肠没有保护作用, 甚至抑酸药会明显加重NSAIDs引起的小肠损伤和出血. 一项研究表明, 奥美拉唑有降低微生物多样性、操作分类单元(operational taxonomic units, OTU)、菌群丰度指数(Chao1指数)和菌群多样性指数(Shannon指数)的趋势. 同样地, Wang等人[27]的研究表明, PPI是低剂量阿司匹林引起的小肠损伤的独立危险因素. 此外, 有研究发现[28], 雷贝拉唑会增加健康志愿者服用塞来昔布后小肠损伤的发生率. 这些结果提示, PPI可导致小肠微生物多样性丧失, 这可能是PPI加重NSAIDs所致小肠损伤的重要原因.
小肠细菌过度生长(small intestinal bacterial overgrowth, SIBO)是一种菌群失调, 表现为小肠中细菌过多, 空肠中细菌大于105个单位/毫升个菌落, 并出现症状或吸收不良[29]. 十二指肠抽吸术(duodenal aspiration, DA)和乳果糖呼气试验(lactulose breath test, LBT)常用于诊断SIBO. 一项评估SIBO与小肠损伤之间关系的研究显示[30], 通过LBT诊断, SIBO是NSAIDs和长期低剂量阿司匹林使用者发生严重小肠损伤的独立危险因素. 此外, 解剖异常[31]、小肠动力障碍[32]等都可能会导致SIBO.
研究发现, 联合使用非阿司匹林类NSAIDs比单独使用阿司匹林对小肠的粘膜损伤程度更重[33]. 一项临床研究调查显示[34], 在服用消炎痛后, 男性的肠道通透性高于女性, 且微生物多样性低于女性. 这些变化在停药后可恢复正常. 在NSAIDs引起的小肠损伤中, 老年人群中的发病率更高[35], 并且随着年龄的增长, 每年可增加4%的发病风险[36]. 此外, 心理应激会引起肠道微生物群和通透性的变化, 从而加剧NSAIDs引起的小肠损伤[37]. 另外, 食用含乳化剂聚山梨酯-80(polysorbate 80, p80)的食品可导致回肠菌群失调, 增强了NSAIDs诱导的小肠损伤[38]. 这些针对危险因素的研究只涉及少数患者, 因此, 还需要进行大规模的临床研究来进一步证实.
与NSAIDs引起上消化道损伤的机制不同, NSAIDs相关小肠损伤的发病机制尚未明确. 目前, 普遍认为NSAIDs诱导的肠病是一个多因素、多步骤协同导致的病理损伤.
在小肠中, 环氧合酶1(cyclo-oxygen-ase-1, COX-1)负责黏膜前列腺素的合成, 前列腺素E2(prostaglandin E2, PGE2)是小肠中主要的前列腺素, 负责粘液和碳酸氢盐的合成和分泌, 以及调节黏膜血流和上皮细胞增殖, 而NSAIDs可抑制环氧化酶的活性. 最初, NSAIDs相关肠病的发病机制认为只与COX-1受到抑制有关. 然而, Hotz-Behofsits等人[39]证明了选择性COX-1抑制不会导致小肠损害, 联合抑制COX-1和环氧合酶2(cyclo-oxygen-ase-2, COX-2)或抑制COX-2会导致肠局部损伤, 这表明, NSAIDs肠病可以在没有COX-1抑制的情况下发生, COX-1介导产生的PGE2不足以保护NSAIDs引起的局部肠损伤.
NSAIDs可以抑制肠内前列腺素的合成, 但缺乏前列腺素并不一定会导致小肠损伤[40]. NSAIDs局部损伤作用是通过非环氧化酶依赖途径, 是药物在肠腔内与黏膜接触的结果[41,42], 主要涉及NSAIDs对肠上皮细胞内线粒体的影响. 在实验中发现[43], NSAIDs可以解除离体大鼠肝线粒体的氧化磷酸化, 并抑制了偶联线粒体的呼吸作用. NSAIDs的解偶联活性作用主要是由于线粒体膜上转换孔(mitochondrial permeability transition pore, mPTP)的开放, PTP是由连接线粒体膜内外的蛋白质组成[44-46]. mPTP的开放与线粒体功能障碍有关[47]. mPTP的开放可以诱导细胞色素c从线粒体基质释放到细胞质中, 从而导致细胞死亡. 因此, 线粒体损伤可能引起黏膜屏障功能紊乱和小肠通透性升高, 并且在NSAIDs引起的小肠损伤的早期过程中起着重要的作用[42].
胆汁在小肠损伤的发病机制中起着重要作用.熊去氧胆酸加重了吲哚美辛引起的大鼠肠道炎症, 在一项肠道鼠肠上皮细胞(intestinal epithelial cell-6, IEC-6)的体外研究中, 胆汁和吲哚美辛的联合使用增加了细胞膜的通透性, 并且比单独使用这些药物细胞毒性更强. 另一项研究发现, 给大鼠服用NSAIDs会增加了胆汁中次级胆汁酸的浓度[48], 次级胆汁酸对肠上皮细胞毒性比初级胆汁酸更强.初级胆汁酸向次级胆汁酸的转化依赖于肠道细菌酶, 因此, NSAIDs可能通过改变了肠道微生物从而改变胆汁的细胞毒性. 虽然胆汁酸损伤小肠的确切机制尚不清楚, 但某些胆汁酸, 如牛磺脱氧胆酸和脱氧胆酸, 已被证实在IEC-6细胞中能诱导促炎因子IL-8的产生, 并激活NF-κΒ信号通路[49,50]. 另外, 有研究显示, 鹅去氧胆酸等胆汁酸可以打开PTP通道从而引起肠上皮细胞死亡[51].
胆汁介导的小肠损伤可能有两种机制. 首先, 包括胆汁酸在内的胆汁成分是NSAIDs所致肠病发病的主要侵袭性因素. 其次, 肠-肝循环在小肠损伤的发病机制中起着至关重要的作用. 许多NSAIDs具有羧酸结构, 在肝脏中与酰基葡萄糖醛酸结合后分泌到胆汁中. 这些偶联物经小肠管腔中的细菌β-葡萄糖醛酸酶酶解后被重新吸收. 这种肠-肝循环可导致肠粘膜长期和反复暴露于NSAIDs, 导致肠道损伤. 未经过肠-肝循环的NSAIDs则没有引起明显的小肠溃疡[52,53].
肠道细菌在NSAIDs引起的小肠溃疡中起着至关重要的作用. Robert等人[54]报道, 用吲哚美辛治疗的无菌大鼠未出现肠溃疡, 而暴露于大肠杆菌的无菌大鼠, 大肠杆菌对小肠产生了严重的损伤. 研究发现[55,56], 在NSAIDs引起小肠损伤的发生过程中, 革兰氏阴性细菌数量增加. 而在给予氨苄青霉素后, 能显著抑制NSAIDs诱导的小肠损伤[57]. 尽管肠道细菌侵入粘膜的机制目前尚不清楚, 但研究表明[58-60], 在吲哚美辛治疗后, 粘液分泌的减少可能是导致损伤的原因之一. 粘液在抵抗肠道病原体和刺激物中起着重要的作用, 粘液分泌的减少可能会削弱肠道屏障, 导致细菌入侵. 一项研究发现[60], 氯索洛芬降低了小肠MUC2mRNA的表达, MUC2是一种重要的粘蛋白, 在分泌型粘蛋白的二聚化过程中起主要作用, 这是胃肠道粘液形成的关键步骤[61]. 综上, 肠道细菌损伤肠粘膜是通过多种因素实现的, 包括肠道运动亢进和诱导型一氧化氮合酶/一氧化氮的上调[40].
肠道内先天免疫的激活, 主要是通过肠道细菌和其他因素触发了炎症级联反应.
Toll样受体(toll-like receptors, TLR)家族在针对微生物病原体的先天免疫应答以及随后的适应性免疫应答中起着至关重要的作用. TLR能识别微生物病原体的特定分子模式-病原体相关分子模式(pathogen-associated molecular patterns, PAMPs). 这些外源性配体与TLR结合激活下游信号通路, 包括核因子κB(NF-κB)、丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)和Ⅰ型干扰素(IFN-I)通路, 诱导促炎细胞因子和趋化因子产生, 加重肠道损伤. TLR除了识别PAMPs之外, 也能够识别内源性配体-危险相关分子模式(danger-associated molecular patters, DAMPs). 高迁移率族蛋白盒-1(high mobility group box-1 protein, HMGB-1), 从损伤的上皮细胞释放, 通过TLR2和晚期糖基化终末产物受体(receptor for advanced glycation end products, RAGE)以及TLR4发挥促炎作用[62]. 有研究发现[63], 在NSAIDs诱导的肠病中, 重组HMGB-1也可以通过激活NF-κB和MAPK通路加重肠道损伤.
NSAIDs引发的炎症信号还能够激活NLR家族[样受体家族3(NOD-like receptors, NLRP3)]的炎症体[64], NLRP3可以识别细胞内外产生的应激源信号, 触发炎症小体的组装, 导致半胱氨酸天冬氨酸蛋白酶-1(caspase-1)原的激活[65]. Caspase-1被激活后, 可以促进白介素-1β前体(Pro-IL-1β)和白介素-18前体(Pro-IL-18)的加工, 使它们变成活性形式. 白介素-1β(IL-1β)可以加重NSAIDs引起的肠道损伤. 此外, 研究发现[64], NLRP3和caspase-1基因缺陷型小鼠的小肠损伤严重程度减轻, 同时成熟的IL-1β的合成水平降低, 提示NLRP3衍生的IL-1β介导了炎症级联反应和损伤.
对于NSAIDs引起的复杂肠病, 一项回顾性研究显示, 停用NSAIDs后, 71%的患者, 在随访15.9个月后, 经内窥镜检查, 仅有4.3%出现并发症[8].故可先行保守治疗, 如出现并发症, 采用药物治疗干预.
预防NSAIDs相关肠病最有效的方法是停用NSAIDs, 然而, 某些心血管疾病患者, 禁止停用NSAIDs. 因此, 通过药物防治是安全使用NSAIDs的主要措施(表1).
| 黏膜保护剂 | 益生菌 | 抗生素 | 新型组胺H2受体拮抗剂 | 营养干预 | 其他 |
| 米索前列醇 | 干酪乳杆菌 | 甲硝唑 | 拉夫替丁 | 谷氨酰胺 | TLR2激动剂 |
| 瑞巴派特 | 加氏乳杆菌 | 利福昔明 | 芦荟 | 云母 | |
| 替普瑞酮 | 短双歧杆菌Bif195 | 牛初乳 | 柚皮苷 | ||
| 干酪乳杆菌菌株Shirota | 重组人乳铁蛋白 | ||||
| 青春型双歧杆菌 | 鸡蛋 | ||||
| 干酪乳杆菌CRL431 | 太平洋鳕鱼 | ||||
| 副干酪乳杆菌CNCM I-1518 | 木瓜 | ||||
| 植物乳杆菌TIFN101和WCFS1 | |||||
| 双歧杆菌BB536 |
6.2.1 黏膜保护剂: 米索前列醇: 前列腺素(prostaglandinum, PG)在黏膜保护中起着重要作用. 在多项研究中, 合成的PG类似物米索前列醇可以降低NSAIDs引起的肠道通透性增加[35,66]. 尽管米索前列醇对NSAIDs引起的肠病有治疗效果, 但它也会引起许多胃肠道不良反应, 如恶心、消化不良、腹痛和腹泻[12]. 这些副作用可能限制其在临床上的应用.
瑞巴派特: 瑞巴派特是一种粘膜保护药物, 已在临床上用于治疗胃炎和消化性溃疡[67]. 临床证据表明[68], 瑞巴派特对NSAIDs引起的小肠损伤有保护作用. 瑞巴派特不仅可以通过上调COX-2的表达和β-catenin的合成来改善小肠的屏障结构[69], 还可以增加小肠粘液分泌从而减轻NSAIDs对小肠的损伤[70]. 此外, 有研究显示[71,72], 瑞巴派特可以通过调节肠道菌群, 抑制吲哚美辛诱导的小肠损伤. 因此, 在临床上, 瑞巴派特可能有助于减轻服用NSAIDs的患者的小肠损伤.
替普瑞酮: 替普瑞酮是一种副作用较小的粘膜保护剂, 在亚洲地区广泛用于治疗胃炎和胃溃疡. 已有报道[73,74], 替普瑞酮不仅对NSAIDs引起的胃粘膜损伤有保护作用, 而且对小肠粘膜损伤也有保护作用. 有研究发现[75], 替普瑞酮可以通过减轻黏膜的自由基损伤以及促进小肠中血管内皮生长因子的合成, 改善阿司匹林导致的小肠黏膜损伤.
6.2.2 益生菌: 益生菌对NSAIDs诱导的肠病的防治十分重要. 研究显示[76,77], 干酪乳杆菌和加氏乳杆菌对长期低剂量阿司匹林使用者的肠病有显著的保护作用. 口服短双歧杆菌Bif195可降低健康志愿者使用阿司匹林所引起的小肠肠病的风险[78]. 但这些益生菌的保护机制尚不清楚. NSAIDs联合PPI的使用促进了阿克曼菌的生长并抑制了双歧杆菌的生长, 引起小肠中的杯状细胞数量减少, 使粘液层变薄, 引起小肠损伤, 而双歧杆菌G9-1可以通过减轻这种损伤来保护小肠[79]. 除了对肠道细菌的直接作用外, 益生菌代谢物的抗炎特性也有助于抑制NSAIDs诱导的小肠损伤. 研究发现[80], 这与干酪乳杆菌菌株Shirota(LcS)产生的乳酸阻止了脂多糖触发巨噬细胞中NF-κB和MAPK的激活有关. 青春型双歧杆菌, 因其有分泌高水平乳酸的能力, 在治疗萘普生引起的小肠溃疡和出血中非常有效[81]. 另外, 在吲哚美辛相关肠病中, 益生菌(干酪乳杆菌CRL431、副干酪乳杆菌CNCM I-1518)通过增强抗菌活性和增加潘氏细胞数量, 增强了肠道屏障功能, 同时, 促进了调节性T细胞分泌IL-10, 抑制肠道炎症, 避免了NSAIDs诱导的小肠损伤[82]. 另有研究显示[83], 口服植物乳杆菌TIFN101和WCFS1可以下调小肠的抗原呈递途径, 防止了由NSAIDs引起的小肠的免疫应激. 此外, 双氯芬酸诱导大鼠肠道损伤时, 联合使用乳铁蛋白与双歧杆菌BB536可以降低肠道损伤, 并恢复髓过氧化物酶(myeloperoxidase, MPO)和血红蛋白水平, 这些可能是通过调节TLR-2/-4/NF-κB途径而实现的[84]. 因此, 益生菌是治疗小肠损伤的一种很有前途的方法.
6.2.3 抗生素: 甲硝唑与NSAIDs联合使用可有效减轻NSAIDs所致肠病[85]. 甲硝唑减轻了NSAIDs引起的肠道炎症和失血, 但不影响肠道渗透性. 甲硝唑的保护作用不是通过抑制肠道内细菌, 而是通过抑制肠上皮细胞线粒体中的氧化磷酸化来实现的[12]. 利福昔明也可以用于治疗NSAIDs相关小肠损伤, 其不仅可以直接抗菌, 还可以减少细菌毒力, 减少细菌对上皮细胞的黏附力、调节肠道免疫信号[86]. 有研究显示[87], 利福昔明可以抑制吲哚美辛引起的大鼠小肠中MPO的活性升高和TNF-α的表达, 并调节肠道菌群. 此外, 研究发现[88], 利福昔明对健康志愿者服用两周双氯芬酸后引起的肠道损伤具有预防保护作用.
6.2.4 新型组胺H2受体拮抗剂: 拉夫替丁作为一种新型组胺H2受体拮抗药,不仅能持久地抑制胃酸分泌, 还能通过激活辣椒素敏感传入神经(capsaicin-sensitive afferent neurons, CSAN), 促进降钙素基因相关肽和生长抑素释放, 增加胃黏膜血流量, 促进黏膜上皮再生, 增强消化道黏膜屏障[89,90]. 有研究发现[60], 拉夫替丁可以通过介导CSN, 增加小肠Muc2的表达和黏液的分泌, 抑制细菌入侵和诱导型一氧化氮合酶表达, 从而保护NSAIDs对小肠的损害. 拉呋替丁既能抑制胃酸分泌保护胃黏膜, 又可通过激活CSN保护小肠黏膜, 有望成为理想的"NSAIDs伴侣", 但仍需进一步的临床研究验证[91].
6.2.5 营养干预: 营养治疗与药物治疗相比, 药理风险相对较低. 谷氨酰胺是一种非必需氨基酸, 作为肠粘膜细胞的能量来源, 据报道[92], 短期服用NSAIDs后, 谷氨酰胺可有效预防肠粘膜通透性增高. 加工过的芦荟凝胶(processed Aloe vera gel, PAG)在临床上有很多益处, 如促进伤口愈合、抗癌、抗氧化、抗菌作用. 研究发现[93], PAG可以通过ERK依赖途径, 调节小肠细胞中粘蛋白的表达, 从而减轻NSAIDs所致小肠溃疡. 牛初乳中含有大量的生长因子, 如胰岛素样生长因子、各种免疫球蛋白和抗菌肽. 食用牛初乳可有效减少短期使用NSAIDs引起的肠道损伤和细菌移位[94,95]. 此外, 重组人乳铁蛋白具有杀菌、抗炎和抗氧化作用, 可减轻NSAIDs诱导的小肠通透性增加[94,96]. 通过细胞实验和动物实验发现[97], 鸡蛋可以促进细胞增殖, 减轻NSAIDs引起的小鼠小肠绒毛损伤. 太平洋鳕鱼分解物可以减少NSAIDs引起的小鼠小肠细胞凋亡[98]. 木瓜提取物通过调节肠道组织内质网应激来预防NSAIDs对小鼠小肠黏膜的损伤[99].
6.2.6 其他药物: TLR2激动剂可以通过抑制TLR4信号传导, 减轻吲哚美辛诱导的回肠炎[100]. 云母可以通过ERK信号通路抑制NSAIDs引起的小肠损伤[101]. 柚皮苷可以促进生长素释放肽的分泌和GSH-R的表达, 并抑制TNF-α的释放, 从而修复肠黏膜[102], 这些药物将有希望成为防治NSAIDs相关肠病的新方法.
NSAIDs所致的小肠损伤已成为临床上的一个重要问题. 其具体损伤机制尚未明确, 虽然目前发现一些药物可以对NSAIDs相关小肠损伤起保护作用, 但还没有确切的临床试验支持. 因此, 仍需要对服用NSAIDs的患者进行长期研究, 加强对NSAIDs相关小肠损伤的防治.
学科分类: 胃肠病学和肝病学
手稿来源地: 天津市
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): B, B, B
C级 (良好): 0
D级 (一般): 0
E级 (差): 0
科学编辑: 张砚梁 制作编辑:张砚梁
| 1. | Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol Drug Saf. 2014;23:43-50. [PubMed] [DOI] |
| 2. | Bertrand KA, Bethea TN, Gerlovin H, Coogan PF, Barber L, Rosenberg L, Palmer JR. Aspirin use and risk of breast cancer in African American women. Breast Cancer Res. 2020;22:96. [PubMed] [DOI] |
| 3. | Zhao M, Wang Y, Du C, Liu Y, Zhang N, Luo F. Aspirin and metformin exhibit antitumor activity in murine breast cancer. Oncol Rep. 2018;39:1414-1422. [PubMed] [DOI] |
| 4. | Albandar HJ, Markert R, Agrawal S. The relationship between aspirin use and mortality in colorectal cancer. J Gastrointest Oncol. 2018;9:1133-1137. [PubMed] [DOI] |
| 5. | Chan FK, Kyaw M, Tanigawa T, Higuchi K, Fujimoto K, Cheong PK, Lee V, Kinoshita Y, Naito Y, Watanabe T, Ching JY, Lam K, Lo A, Chan H, Lui R, Tang RS, Sakata Y, Tse YK, Takeuchi T, Handa O, Nebiki H, Wu JC, Abe T, Mishiro T, Ng SC, Arakawa T. Similar Efficacy of Proton-Pump Inhibitors vs H2-Receptor Antagonists in Reducing Risk of Upper Gastrointestinal Bleeding or Ulcers in High-Risk Users of Low-Dose Aspirin. Gastroenterology. 2017;152:105-110.e1. [PubMed] [DOI] |
| 6. | May A. Double-Balloon Enteroscopy. Gastrointest Endosc Clin N Am. 2017;27:113-122. [PubMed] [DOI] |
| 7. | Xu N, Yu Z, Cao X, Wang Z, Yan M. Characteristics of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)-Induced Small Bowel Injury Identified by Single-Balloon Endoscopy or Capsule Endoscopy. Med Sci Monit. 2017;23:5237-5245. [PubMed] [DOI] |
| 8. | Shim KN, Song EM, Jeen YT, Kim JO, Jeon SR, Chang DK, Song HJ, Lim YJ, Kim JS, Ye BD, Park CH, Jeon SW, Cheon JH, Lee KJ, Kim JH, Jang BI, Moon JS, Chun HJ, Choi MG; Korean Gut Image Study Group. Long-Term Outcomes of NSAID-Induced Small Intestinal Injury Assessed by Capsule Endoscopy in Korea: A Nationwide Multicenter Retrospective Study. Gut Liver. 2015;9:727-733. [PubMed] [DOI] |
| 9. | Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Alonso-Abreu I, Perez-Aisa MA, Perez-Gisbert J, Bujanda L, Castro M, Muñoz M, Rodrigo L, Calvet X, Del-Pino D, Garcia S. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104:1633-1641. [PubMed] [DOI] |
| 10. | Kamil R, Geier MS, Butler RN, Howarth GS. Lactobacillus rhamnosus GG exacerbates intestinal ulceration in a model of indomethacin-induced enteropathy. Dig Dis Sci. 2007;52:1247-1252. [PubMed] [DOI] |
| 11. | Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG; Investigators. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133-141. [PubMed] [DOI] |
| 12. | Fortun PJ, Hawkey CJ. Nonsteroidal antiinflammatory drugs and the small intestine. Curr Opin Gastroenterol. 2007;23:134-141. [PubMed] [DOI] |
| 13. | Maiden L. Capsule endoscopic diagnosis of nonsteroidal antiinflammatory drug-induced enteropathy. J Gastroenterol. 2009;44 Suppl 19:64-71. [PubMed] [DOI] |
| 14. | Park SC, Chun HJ, Kang CD, Sul D. Prevention and management of non-steroidal anti-inflammatory drugs-induced small intestinal injury. World J Gastroenterol. 2011;17:4647-4653. [PubMed] [DOI] |
| 15. | Schiller LR. Lower gastrointestinal complications of nonsteroidal anti-inflammatory drugs. Curr Gastroenterol Rep. 2003;5:397-398. [PubMed] [DOI] |
| 16. | Aabakken L, Osnes M. 51Cr-ethylenediaminetetraacetic acid absorption test. Effects of naproxen, a non-steroidal, antiinflammatory drug. Scand J Gastroenterol. 1990;25:917-924. [PubMed] [DOI] |
| 17. | Davies NM. Sustained release and enteric coated NSAIDs: are they really GI safe? J Pharm Pharm Sci. 1999;2:5-14. [PubMed] |
| 18. | Tibble JA, Sigthorsson G, Foster R, Scott D, Fagerhol MK, Roseth A, Bjarnason I. High prevalence of NSAID enteropathy as shown by a simple faecal test. Gut. 1999;45:362-366. [PubMed] [DOI] |
| 19. | Brito HP, Ribeiro IB, de Moura DTH, Bernardo WM, Chaves DM, Kuga R, Maahs ED, Ishida RK, de Moura ETH, de Moura EGH. Video capsule endoscopy vs double-balloon enteroscopy in the diagnosis of small bowel bleeding: A systematic review and meta-analysis. World J Gastrointest Endosc. 2018;10:400-421. [PubMed] [DOI] |
| 20. | Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172-1178. [PubMed] [DOI] |
| 21. | Adebayo D, Bjarnason I. Is non-steroidal anti-inflammaory drug (NSAID) enteropathy clinically more important than NSAID gastropathy? Postgrad Med J. 2006;82:186-191. [PubMed] [DOI] |
| 22. | Thiéfin G, Beaugerie L. Toxic effects of nonsteroidal antiinflammatory drugs on the small bowel, colon, and rectum. Joint Bone Spine. 2005;72:286-294. [PubMed] [DOI] |
| 23. | Shimada S, Watanabe T, Nadatani Y, Otani K, Taira K, Hosomi S, Nagami Y, Tanaka F, Kamata N, Yamagami H, Tanigawa T, Shiba M, Fujiwara Y. Clinical factors associated with positive capsule endoscopy findings in patients with obscure gastrointestinal bleeding: a single-center study. Scand J Gastroenterol. 2017;52:1219-1223. [PubMed] [DOI] |
| 24. | Blackler R, Syer S, Bolla M, Ongini E, Wallace JL. Gastrointestinal-sparing effects of novel NSAIDs in rats with compromised mucosal defence. PLoS One. 2012;7:e35196. [PubMed] [DOI] |
| 25. | Nadatani Y, Watanabe T, Suda W, Nakata A, Matsumoto Y, Kosaka S, Higashimori A, Otani K, Hosomi S, Tanaka F, Nagami Y, Kamata N, Taira K, Yamagami H, Tanigawa T, Hattori M, Fujiwara Y. Gastric acid inhibitor aggravates indomethacin-induced small intestinal injury via reducing Lactobacillus johnsonii. Sci Rep. 2019;9:17490. [PubMed] [DOI] |
| 26. | Wallace JL, Syer S, Denou E, de Palma G, Vong L, McKnight W, Jury J, Bolla M, Bercik P, Collins SM, Verdu E, Ongini E. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314-1322, 1322.e1-1322.e5. [PubMed] [DOI] |
| 27. | Wang LJ, Xue T, Wu YQ, Zhao JY, Wang TN, Li JT, Fu CL, Ma JJ, Zhang LP, Shao YX, Yang YC, Zhou ZX, Ma HF. [Effect of acupuncture on intestinal flora in rats with stress gastric ulcer]. Zhongguo Zhen Jiu. 2020;40:526-532. [PubMed] [DOI] |
| 28. | Washio E, Esaki M, Maehata Y, Miyazaki M, Kobayashi H, Ishikawa H, Kitazono T, Matsumoto T. Proton Pump Inhibitors Increase Incidence of Nonsteroidal Anti-Inflammatory Drug-Induced Small Bowel Injury: A Randomized, Placebo-Controlled Trial. Clin Gastroenterol Hepatol. 2016;14:809-815.e1. [PubMed] [DOI] |
| 29. | DONALDSON RM. NORMAL BACTERIAL POPULATIONS OF THE INTESTINE AND THEIR RELATION TO INTESTINAL FUNCTION. N Engl J Med. 1964;270:938-45 CONTD. [PubMed] [DOI] |
| 30. | Muraki M, Fujiwara Y, Machida H, Okazaki H, Sogawa M, Yamagami H, Tanigawa T, Shiba M, Watanabe K, Tominaga K, Watanabe T, Arakawa T. Role of small intestinal bacterial overgrowth in severe small intestinal damage in chronic non-steroidal anti-inflammatory drug users. Scand J Gastroenterol. 2014;49:267-273. [PubMed] [DOI] |
| 31. | Alcaraz F, Frey S, Iannelli A. Surgical Management of Small Intestinal Bacterial Overgrowth After Roux-en-Y Gastric Bypass. Obes Surg. 2020;30:4677-4678. [PubMed] [DOI] |
| 32. | Chander Roland B, Mullin GE, Passi M, Zheng X, Salem A, Yolken R, Pasricha PJ. A Prospective Evaluation of Ileocecal Valve Dysfunction and Intestinal Motility Derangements in Small Intestinal Bacterial Overgrowth. Dig Dis Sci. 2017;62:3525-3535. [PubMed] [DOI] |
| 33. | Ishihara M, Ohmiya N, Nakamura M, Funasaka K, Miyahara R, Ohno E, Kawashima H, Itoh A, Hirooka Y, Watanabe O, Ando T, Goto H. Risk factors of symptomatic NSAID-induced small intestinal injury and diaphragm disease. Aliment Pharmacol Ther. 2014;40:538-547. [PubMed] [DOI] |
| 34. | Edogawa S, Peters SA, Jenkins GD, Gurunathan SV, Sundt WJ, Johnson S, Lennon RJ, Dyer RB, Camilleri M, Kashyap PC, Farrugia G, Chen J, Singh RJ, Grover M. Sex differences in NSAID-induced perturbation of human intestinal barrier function and microbiota. FASEB J. 2018;fj201800560R. [PubMed] [DOI] |
| 35. | Fujimori S, Seo T, Gudis K, Ehara A, Kobayashi T, Mitsui K, Yonezawa M, Tanaka S, Tatsuguchi A, Sakamoto C. Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: a pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest Endosc. 2009;69:1339-1346. [PubMed] [DOI] |
| 36. | Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010;24:121-132. [PubMed] [DOI] |
| 37. | Yoshikawa K, Kurihara C, Furuhashi H, Takajo T, Maruta K, Yasutake Y, Sato H, Narimatsu K, Okada Y, Higashiyama M, Watanabe C, Komoto S, Tomita K, Nagao S, Miura S, Tajiri H, Hokari R. Psychological stress exacerbates NSAID-induced small bowel injury by inducing changes in intestinal microbiota and permeability via glucocorticoid receptor signaling. J Gastroenterol. 2017;52:61-71. [PubMed] [DOI] |
| 38. | Furuhashi H, Higashiyama M, Okada Y, Kurihara C, Wada A, Horiuchi K, Hanawa Y, Mizoguchi A, Nishii S, Inaba K, Sugihara N, Watanabe C, Komoto S, Tomita K, Miura S, Hokari R. Dietary emulsifier polysorbate-80-induced small-intestinal vulnerability to indomethacin-induced lesions via dysbiosis. J Gastroenterol Hepatol. 2020;35:110-117. [PubMed] [DOI] |
| 39. | Hotz-Behofsits CM, Walley MJ, Simpson R, Bjarnason IT. COX-1, COX-2 and the topical effect in NSAID-induced enteropathy. Inflammopharmacology. 2003;11:363-370. [PubMed] [DOI] |
| 40. | Takeuchi K, Satoh H. NSAID-induced small intestinal damage--roles of various pathogenic factors. Digestion. 2015;91:218-232. [PubMed] [DOI] |
| 41. | Bjarnason I, Scarpignato C, Holmgren E, Olszewski M, Rainsford KD, Lanas A. Mechanisms of Damage to the Gastrointestinal Tract From Nonsteroidal Anti-Inflammatory Drugs. Gastroenterology. 2018;154:500-514. [PubMed] [DOI] |
| 42. | Watanabe T, Tanigawa T, Nadatani Y, Otani K, Machida H, Okazaki H, Yamagami H, Watanabe K, Tominaga K, Fujiwara Y, Arakawa T. Mitochondrial disorders in NSAIDs-induced small bowel injury. J Clin Biochem Nutr. 2011;48:117-121. [PubMed] [DOI] |
| 43. | Somasundaram S, Rafi S, Hayllar J, Sigthorsson G, Jacob M, Price AB, Macpherson A, Mahmod T, Scott D, Wrigglesworth JM, Bjarnason I. Mitochondrial damage: a possible mechanism of the "topical" phase of NSAID induced injury to the rat intestine. Gut. 1997;41:344-353. [PubMed] [DOI] |
| 44. | Al-Nasser IA. Salicylate-induced kidney mitochondrial permeability transition is prevented by cyclosporin A. Toxicol Lett. 1999;105:1-8. [PubMed] [DOI] |
| 45. | Singh BK, Tripathi M, Pandey PK, Kakkar P. Nimesulide aggravates redox imbalance and calcium dependent mitochondrial permeability transition leading to dysfunction in vitro. Toxicology. 2010;275:1-9. [PubMed] [DOI] |
| 46. | Oh KW, Qian T, Brenner DA, Lemasters JJ. Salicylate enhances necrosis and apoptosis mediated by the mitochondrial permeability transition. Toxicol Sci. 2003;73:44-52. [PubMed] [DOI] |
| 47. | Scatena R, Bottoni P, Botta G, Martorana GE, Giardina B. The role of mitochondria in pharmacotoxicology: a reevaluation of an old, newly emerging topic. Am J Physiol Cell Physiol. 2007;293:C12-C21. [PubMed] [DOI] |
| 48. | Yamada T, Hoshino M, Hayakawa T, Kamiya Y, Ohhara H, Mizuno K, Yamada H, Nakazawa T, Inagaki T, Uchida A, Miyaji M, Takeuchi T. Bile secretion in rats with indomethacin-induced intestinal inflammation. Am J Physiol. 1996;270:G804-G812. [PubMed] [DOI] |
| 49. | Strauch ED, Bass BL, Rao JN, Vann JA, Wang JY. NF-kappaB regulates intestinal epithelial cell and bile salt-induced migration after injury. Ann Surg. 2003;237:494-501. [PubMed] [DOI] |
| 50. | Mühlbauer M, Allard B, Bosserhoff AK, Kiessling S, Herfarth H, Rogler G, Schölmerich J, Jobin C, Hellerbrand C. Differential effects of deoxycholic acid and taurodeoxycholic acid on NF-kappa B signal transduction and IL-8 gene expression in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1000-G1008. [PubMed] [DOI] |
| 51. | Rolo AP, Oliveira PJ, Moreno AJ, Palmeira CM. Chenodeoxycholate is a potent inducer of the permeability transition pore in rat liver mitochondria. Biosci Rep. 2001;21:73-80. [PubMed] [DOI] |
| 52. | Mayo SA, Song YK, Cruz MR, Phan TM, Singh KV, Garsin DA, Murray BE, Dial EJ, Lichtenberger LM. Indomethacin injury to the rat small intestine is dependent upon biliary secretion and is associated with overgrowth of enterococci. Physiol Rep. 2016;4. [PubMed] [DOI] |
| 53. | Yarushkina NI, Filaretova LP. Effects of stress preconditioning on vulnerability of gastric and small intestinal mucosa to ulcerogenic action of indomethacin in rats. J Physiol Pharmacol. 2019;70. [PubMed] [DOI] |
| 54. | Robert A, Asano T. Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins. 1977;14:333-341. [PubMed] [DOI] |
| 55. | Reuter BK, Davies NM, Wallace JL. Nonsteroidal anti-inflammatory drug enteropathy in rats: role of permeability, bacteria, and enterohepatic circulation. Gastroenterology. 1997;112:109-117. [PubMed] [DOI] |
| 56. | Hagiwara M, Kataoka K, Arimochi H, Kuwahara T, Ohnishi Y. Role of unbalanced growth of gram-negative bacteria in ileal ulcer formation in rats treated with a nonsteroidal anti-inflammatory drug. J Med Invest. 2004;51:43-51. [PubMed] [DOI] |
| 57. | Konaka A, Kato S, Tanaka A, Kunikata T, Korolkiewicz R, Takeuchi K. Roles of enterobacteria, nitric oxide and neutrophil in pathogenesis of indomethacin-induced small intestinal lesions in rats. Pharmacol Res. 1999;40:517-524. [PubMed] [DOI] |
| 58. | Kunikata T, Tanaka A, Miyazawa T, Kato S, Takeuchi K. 16, 16-Dimethyl prostaglandin E2 inhibits indomethacin-induced small intestinal lesions through EP3 and EP4 receptors. Dig Dis Sci. 2002;47:894-904. [PubMed] [DOI] |
| 59. | Kamei K, Kubo Y, Kato N, Hatazawa R, Amagase K, Takeuchi K. Prophylactic effect of irsogladine maleate against indomethacin-induced small intestinal lesions in rats. Dig Dis Sci. 2008;53:2657-2666. [PubMed] [DOI] |
| 60. | Amagase K, Ochi A, Sugihara T, Kato S, Takeuchi K. Protective effect of lafutidine, a histamine H2 receptor antagonist, against loxoprofen-induced small intestinal lesions in rats. J Gastroenterol Hepatol. 2010;25 Suppl 1:S111-S118. [PubMed] [DOI] |
| 61. | Takeuchi K, Yokota A, Tanaka A, Takahira Y. Factors involved in upregulation of inducible nitric oxide synthase in rat small intestine following administration of nonsteroidal anti-inflammatory drugs. Dig Dis Sci. 2006;51:1250-1259. [PubMed] [DOI] |
| 62. | Paudel YN, Angelopoulou E, Piperi C, Balasubramaniam VRMT, Othman I, Shaikh MF. Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: Updates on receptor signalling. Eur J Pharmacol. 2019;858:172487. [PubMed] [DOI] |
| 63. | Nadatani Y, Watanabe T, Tanigawa T, Machida H, Okazaki H, Yamagami H, Watanabe K, Tominaga K, Fujiwara Y, Arakawa T. High mobility group box 1 promotes small intestinal damage induced by nonsteroidal anti-inflammatory drugs through Toll-like receptor 4. Am J Pathol. 2012;181:98-110. [PubMed] [DOI] |
| 64. | Higashimori A, Watanabe T, Nadatani Y, Takeda S, Otani K, Tanigawa T, Yamagami H, Shiba M, Tominaga K, Fujiwara Y, Arakawa T. Mechanisms of NLRP3 inflammasome activation and its role in NSAID-induced enteropathy. Mucosal Immunol. 2016;9:659-668. [PubMed] [DOI] |
| 65. | Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;4:198-208. [PubMed] [DOI] |
| 66. | Fujimori S, Takahashi Y, Seo T, Gudis K, Ehara A, Kobayashi T, Mitsui K, Yonezawa M, Tanaka S, Tatsuguchi A, Sakamoto C. Prevention of traditional NSAID-induced small intestinal injury: recent preliminary studies using capsule endoscopy. Digestion. 2010;82:167-172. [PubMed] [DOI] |
| 67. | Kim JH, Park SH, Cho CS, Lee ST, Yoo WH, Kim SK, Kang YM, Rew JS, Park YW, Lee SK, Lee YC, Park W, Lee DH. Preventive efficacy and safety of rebamipide in nonsteroidal anti-inflammatory drug-induced mucosal toxicity. Gut Liver. 2014;8:371-379. [PubMed] [DOI] |
| 68. | Watanabe T, Takeuchi T, Handa O, Sakata Y, Tanigawa T, Shiba M, Naito Y, Higuchi K, Fujimoto K, Yoshikawa T, Arakawa T. A multicenter, randomized, double-blind, placebo-controlled trial of high-dose rebamipide treatment for low-dose aspirin-induced moderate-to-severe small intestinal damage. PLoS One. 2015;10:e0122330. [PubMed] [DOI] |
| 69. | Lai Y, Zhong W, Yu T, Xia ZS, Li JY, Ouyang H, Shan TD, Yang HS, Chen QK. Rebamipide Promotes the Regeneration of Aspirin-Induced Small-Intestine Mucosal Injury through Accumulation of β-Catenin. PLoS One. 2015;10:e0132031. [PubMed] [DOI] |
| 70. | Suyama Y, Handa O, Naito Y, Takayama S, Mukai R, Ushiroda C, Majima A, Yasuda-Onozawa Y, Higashimura Y, Fukui A, Dohi O, Okayama T, Yoshida N, Katada K, Kamada K, Uchiyama K, Ishikawa T, Takagi T, Konishi H, Itoh Y. Mucus reduction promotes acetyl salicylic acid-induced small intestinal mucosal injury in rats. Biochem Biophys Res Commun. 2018;498:228-233. [PubMed] [DOI] |
| 71. | Kurata S, Nakashima T, Osaki T, Uematsu N, Shibamori M, Sakurai K, Kamiya S. Rebamipide protects small intestinal mucosal injuries caused by indomethacin by modulating intestinal microbiota and the gene expression in intestinal mucosa in a rat model. J Clin Biochem Nutr. 2015;56:20-27. [PubMed] [DOI] |
| 72. | Tanigawa T, Watanabe T, Otani K, Nadatani Y, Ohkawa F, Sogawa M, Yamagami H, Shiba M, Watanabe K, Tominaga K, Fujiwara Y, Takeuchi K, Arakawa T. Rebamipide inhibits indomethacin-induced small intestinal injury: possible involvement of intestinal microbiota modulation by upregulation of α-defensin 5. Eur J Pharmacol. 2013;704:64-69. [PubMed] [DOI] |
| 73. | Umegaki E, Kuramoto T, Kojima Y, Nouda S, Ishida K, Takeuchi T, Inoue T, Tokioka S, Higuchi K. Geranylgeranylacetone, a gastromucoprotective drug, protects against NSAID-induced esophageal, gastroduodenal and small intestinal mucosal injury in healthy subjects: A prospective randomized study involving a comparison with famotidine. Intern Med. 2014;53:283-290. [PubMed] [DOI] |
| 74. | Xiong L, Huang X, Li L, Yang X, Liang L, Zhan Z, Ye Y, Chen M. Geranylgeranylacetone protects against small-intestinal injuries induced by diclofenac in patients with rheumatic diseases: a prospective randomized study. Dig Liver Dis. 2015;47:280-284. [PubMed] [DOI] |
| 76. | Suzuki T, Masui A, Nakamura J, Shiozawa H, Aoki J, Nakae H, Tsuda S, Imai J, Hideki O, Matsushima M, Mine T, Tamura A, Ohtsu T, Asami Y, Takagi A. Yogurt Containing Lactobacillus gasseri Mitigates Aspirin-Induced Small Bowel Injuries: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Digestion. 2017;95:49-54. [PubMed] [DOI] |
| 77. | Endo H, Higurashi T, Hosono K, Sakai E, Sekino Y, Iida H, Sakamoto Y, Koide T, Takahashi H, Yoneda M, Tokoro C, Inamori M, Abe Y, Nakajima A. Efficacy of Lactobacillus casei treatment on small bowel injury in chronic low-dose aspirin users: a pilot randomized controlled study. J Gastroenterol. 2011;46:894-905. [PubMed] [DOI] |
| 78. | Mortensen B, Murphy C, O'Grady J, Lucey M, Elsafi G, Barry L, Westphal V, Wellejus A, Lukjancenko O, Eklund AC, Nielsen HB, Baker A, Damholt A, van Hylckama Vlieg JET, Shanahan F, Buckley M. Bifidobacteriumbreve Bif195 Protects Against Small-Intestinal Damage Caused by Acetylsalicylic Acid in Healthy Volunteers. Gastroenterology. 2019;157:637-646.e4. [PubMed] [DOI] |
| 79. | Yoshihara T, Oikawa Y, Kato T, Kessoku T, Kobayashi T, Kato S, Misawa N, Ashikari K, Fuyuki A, Ohkubo H, Higurashi T, Tateishi Y, Tanaka Y, Nakajima S, Ohno H, Wada K, Nakajima A. The protective effect of Bifidobacterium bifidum G9-1 against mucus degradation by Akkermansia muciniphila following small intestine injury caused by a proton pump inhibitor and aspirin. Gut Microbes. 2020;11:1385-1404. [PubMed] [DOI] |
| 80. | Watanabe T, Nishio H, Tanigawa T, Yamagami H, Okazaki H, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N, Asahara T, Nomoto K, Higuchi K, Takeuchi K, Arakawa T. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: involvement of lactic acid. Am J Physiol Gastrointest Liver Physiol. 2009;297:G506-G513. [PubMed] [DOI] |
| 81. | Syer SD, McKnight W, Aucouturier A, Martin R, Langella P, Wallace JL. Su1724 Bifidobacteria Exert a Protective Effect Against NSAID-Induced Enteropathy That is Dependent on Lactate Production. Gastroenterology. 2012;142. |
| 82. | Monteros MJM, Galdeano CM, Balcells MF, Weill R, De Paula JA, Perdigón G, Cazorla SI. Probiotic lactobacilli as a promising strategy to ameliorate disorders associated with intestinal inflammation induced by a non-steroidal anti-inflammatory drug. Sci Rep. 2021;11:571. [PubMed] [DOI] |
| 83. | de Vos P, Mujagic Z, de Haan BJ, Siezen RJ, Bron PA, Meijerink M, Wells JM, Masclee AAM, Boekschoten MV, Faas MM, Troost FJ. Lactobacillus plantarum Strains Can Enhance Human Mucosal and Systemic Immunity and Prevent Non-steroidal Anti-inflammatory Drug Induced Reduction in T Regulatory Cells. Front Immunol. 2017;8:1000. [PubMed] [DOI] |
| 84. | Fornai M, Pellegrini C, Benvenuti L, Tirotta E, Gentile D, Natale G, Ryskalin L, Colucci R, Piccoli E, Ghelardi E, Blandizzi C, Antonioli L. Protective effects of the combination Bifidobacterium longum plus lactoferrin against NSAID-induced enteropathy. Nutrition. 2020;70:110583. [PubMed] [DOI] |
| 85. | Higuchi K, Umegaki E, Watanabe T, Yoda Y, Morita E, Murano M, Tokioka S, Arakawa T. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879-888. [PubMed] [DOI] |
| 87. | Fornai M, Antonioli L, Pellegrini C, Colucci R, Sacco D, Tirotta E, Natale G, Bartalucci A, Flaibani M, Renzulli C, Ghelardi E, Blandizzi C, Scarpignato C. Small bowel protection against NSAID-injury in rats: Effect of rifaximin, a poorly absorbed, GI targeted, antibiotic. Pharmacol Res. 2016;104:186-196. [PubMed] [DOI] |
| 88. | Scarpignato C, Dolak W, Lanas A, Matzneller P, Renzulli C, Grimaldi M, Zeitlinger M, Bjarnason I. Rifaximin Reduces the Number and Severity of Intestinal Lesions Associated With Use of Nonsteroidal Anti-Inflammatory Drugs in Humans. Gastroenterology. 2017;152:980-982.e3. [PubMed] [DOI] |
| 89. | Ichikawa T, Ota H, Sugiyama A, Maruta F, Ikezawa T, Hotta K, Ishihara K. Effects of a novel histamine H2-receptor antagonist, lafutidine, on the mucus barrier of human gastric mucosa. J Gastroenterol Hepatol. 2007;22:1800-1805. [PubMed] [DOI] |
| 90. | Sugiyama T, Hatanaka Y, Iwatani Y, Jin X, Kawasaki H. Lafutidine facilitates calcitonin gene-related peptide (CGRP) nerve-mediated vasodilation via vanilloid-1 receptors in rat mesenteric resistance arteries. J Pharmacol Sci. 2008;106:505-511. [PubMed] [DOI] |
| 92. | Basivireddy J, Jacob M, Balasubramanian KA. Oral glutamine attenuates indomethacin-induced small intestinal damage. Clin Sci (Lond). 2004;107:281-289. [PubMed] [DOI] |
| 93. | Kim MW, Kang JH, Shin E, Shim KS, Kim MJ, Lee CK, Yoon YS, Oh SH. Processed Aloe vera gel attenuates non-steroidal anti-inflammatory drug (NSAID)-induced small intestinal injury by enhancing mucin expression. Food Funct. 2019;10:6088-6097. [PubMed] [DOI] |
| 94. | Cairangzhuoma, Yamamoto M, Muranishi H, Inagaki M, Uchida K, Yamashita K, Saito S, Yabe T, Kanamaru Y. Skimmed, sterilized, and concentrated bovine late colostrum promotes both prevention and recovery from intestinal tissue damage in mice. J Dairy Sci. 2013;96:1347-1355. [PubMed] [DOI] |
| 96. | Troost FJ, Saris WH, Brummer RJ. Recombinant human lactoferrin ingestion attenuates indomethacin-induced enteropathy in vivo in healthy volunteers. Eur J Clin Nutr. 2003;57:1579-1585. [PubMed] [DOI] |
| 97. | Playford RJ, Garbowsky M, Marchbank T. Pasteurized Chicken Egg Powder Stimulates Proliferation and Migration of AGS, RIE1, and Caco-2 Cells and Reduces NSAID-Induced Injury in Mice and Colitis in Rats. J Nutr. 2020;150:1434-1442. [PubMed] [DOI] |
| 98. | Marchbank T, Elia G, Playford RJ. Intestinal protective effect of a commercial fish protein hydrolysate preparation. Regul Pept. 2009;155:105-109. [PubMed] [DOI] |
| 99. | 郭 冲, 黄 可可, 曾 俊豪, 康 佳敏, 邓 昊, 魏 承亮, 赵 宗尧, 刘 朝奇. 木瓜提取物预防非甾体抗炎药诱导的小鼠肠黏膜损伤. 现代食品科技. 2019;35:45-51. [DOI] |
| 100. | Narimatsu K, Higashiyama M, Kurihara C, Takajo T, Maruta K, Yasutake Y, Sato H, Okada Y, Watanabe C, Komoto S, Tomita K, Nagao S, Miura S, Hokari R. Toll-like receptor (TLR) 2 agonists ameliorate indomethacin-induced murine ileitis by suppressing the TLR4 signaling. J Gastroenterol Hepatol. 2015;30:1610-1617. [PubMed] [DOI] |
| 101. | Zhang S, He Y, Shi Z, Jiang J, He B, Xu S, Fang Z. Small Intestine Protection of Mica Against Non-Steroidal Anti-Inflammatory Drugs-Injury Through ERK1/2 Signal Pathway in Rats. Front Pharmacol. 2019;10:871. [PubMed] [DOI] |